| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:46 UTC |
|---|
| Update date | 2017-01-19 02:36:31 UTC |
|---|
| FoodComEx ID | PC000648 |
|---|
| FoodDB Record | FDB022584 |
|---|
| Chemical Information |
|---|
| Name | Thiamine pyrophosphate |
|---|
| Description | Thiamine pyrophosphate, also known as thiamin diphosphoric acid or THPP, belongs to the class of organic compounds known as thiamine phosphates. These are thiamine derivatives in which the hydroxyl group of the ethanol moiety is substituted by a phosphate group. A 1,3-thiazolium cation that is the conjugate acid of thiamine(1+) diphosphate(1). Thiamine pyrophosphate is a very strong basic compound (based on its pKa). Thiamine pyrophosphate exists in all living species, ranging from bacteria to humans. Within humans, thiamine pyrophosphate participates in a number of enzymatic reactions. In particular, S-(2-methylbutanoyl)-dihydrolipoamide and thiamine pyrophosphate can be biosynthesized from lipoamide and 2-methyl-1-hydroxypropyl-THPP through the action of the enzymes 2-oxoisovalerate dehydrogenase subunit alpha, mitochondrial and 2-oxoisovalerate dehydrogenase subunit beta, mitochondrial. In addition, ketoleucine and thiamine pyrophosphate can be converted into 3-methyl-1-hydroxybutyl-THPP; which is catalyzed by the enzyme 2-oxoisovalerate dehydrogenase. In humans, thiamine pyrophosphate is involved in the metabolic disorder called the 2-methyl-3-hydroxybutyryl-coa dehydrogenase deficiency pathway. |
|---|
| CAS Number | 154-87-0 |
|---|
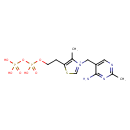
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| Thaimine pyroate | HMDB | | Thaimine pyrophosphate | hmdb | | Thiamin diate | ChEBI | | Thiamin diic acid | Generator | | thiamin diphosphate | hmdb | | Thiamin pyroate | ChEBI | | Thiamin pyroic acid | Generator | | thiamin pyrophosphate | hmdb | | thiamin-PPi | hmdb | | Thiamine diate | ChEBI | | Thiamine diic acid | Generator | | thiamine diphosphate | hmdb | | Thiamine pyroate | HMDB | | Thiamine pyrophosphate | hmdb | | Thiamine pyrophosphic acid | hmdb | | thiamine-PPi | hmdb | | Thiamine-pyroate | HMDB | | thiamine-pyrophosphate | hmdb | | ThPP | hmdb | | TPP | hmdb |
|
|---|
| Chemical Formula | C12H19N4O7P2S |
|---|
| IUPAC name | 3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-5-(2-{[hydroxy(phosphonooxy)phosphoryl]oxy}ethyl)-4-methyl-1,3-thiazol-3-ium |
|---|
| InChI Identifier | InChI=1S/C12H18N4O7P2S/c1-8-11(3-4-22-25(20,21)23-24(17,18)19)26-7-16(8)6-10-5-14-9(2)15-12(10)13/h5,7H,3-4,6H2,1-2H3,(H4-,13,14,15,17,18,19,20,21)/p+1 |
|---|
| InChI Key | AYEKOFBPNLCAJY-UHFFFAOYSA-O |
|---|
| Isomeric SMILES | CC1=C(CCO[P@](O)(=O)OP(O)(O)=O)SC=[N+]1CC1=CN=C(C)N=C1N |
|---|
| Average Molecular Weight | 425.314 |
|---|
| Monoisotopic Molecular Weight | 425.044967696 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as thiamine phosphates. These are thiamine derivatives in which the hydroxyl group of the ethanol moiety is substituted by a phosphate group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Thiamine phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thiamine-phosphate
- Organic pyrophosphate
- 4,5-disubstituted 1,3-thiazole
- Monoalkyl phosphate
- Hydropyrimidine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Imidolactam
- Thiazole
- Azole
- Heteroaromatic compound
- Azacycle
- Organic oxide
- Organic nitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic cation
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 100 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | M204 |
|---|
| Cayman Chemical | 20254 |
|---|
| Glentham | GV8106 |
|---|
| MetaSci | HMDB0001372 |
|---|
| Sigma-Aldrich | HMDB0001372 |
|---|
| Toronto Research Chemicals | T344070 |
|---|