| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:44 UTC |
|---|
| Update date | 2017-01-19 02:36:31 UTC |
|---|
| FoodComEx ID | PC000640 |
|---|
| FoodDB Record | FDB022731 |
|---|
| Chemical Information |
|---|
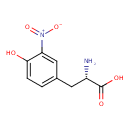
| Name | 3-Nitrotyrosine |
|---|
| Description | 3-Nitrotyrosine (NTyr) is formed in vivo in tissue or blood proteins after exposure to nitrosating and/or nitrating agents such as tetranitromethane. (PMID 8319651)
Reactive nitrogen species such as peroxynitrite can nitrate specific amino acids, whether free or protein bound, and 3-nitrotyrosine is believed to be one marker of this reaction. (PMID 10833032) [HMDB] |
|---|
| CAS Number | 3604-79-3 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2S)-2-amino-3-(4-Hydroxy-3-nitrophenyl)propanoate | Generator | | (2S)-2-amino-3-(4-Hydroxy-3-nitrophenyl)propanoic acid | ChEBI | | 3-Nitrotyrosine | hmdb | | 5-Nitrotyrosine | hmdb | | L-3-Nitrotyrosine | ChEBI | | m-Nitrotyrosine | hmdb |
|
|---|
| Chemical Formula | C9H10N2O5 |
|---|
| IUPAC name | (2S)-2-amino-3-(4-hydroxy-3-nitrophenyl)propanoic acid |
|---|
| InChI Identifier | InChI=1S/C9H10N2O5/c10-6(9(13)14)3-5-1-2-8(12)7(4-5)11(15)16/h1-2,4,6,12H,3,10H2,(H,13,14)/t6-/m0/s1 |
|---|
| InChI Key | FBTSQILOGYXGMD-LURJTMIESA-N |
|---|
| Isomeric SMILES | N[C@@H](CC1=CC=C(O)C(=C1)[N+]([O-])=O)C(O)=O |
|---|
| Average Molecular Weight | 226.1861 |
|---|
| Monoisotopic Molecular Weight | 226.05897144 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as tyrosine and derivatives. Tyrosine and derivatives are compounds containing tyrosine or a derivative thereof resulting from reaction of tyrosine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Tyrosine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tyrosine or derivatives
- Phenylalanine or derivatives
- 3-phenylpropanoic-acid
- Alpha-amino acid
- Amphetamine or derivatives
- Nitrophenol
- L-alpha-amino acid
- Nitrobenzene
- Nitroaromatic compound
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Aralkylamine
- Monocyclic benzene moiety
- Benzenoid
- C-nitro compound
- Amino acid
- Organic nitro compound
- Propargyl-type 1,3-dipolar organic compound
- Allyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxoazanium
- Organic zwitterion
- Organonitrogen compound
- Amine
- Organic oxide
- Organic nitrogen compound
- Organooxygen compound
- Primary aliphatic amine
- Hydrocarbon derivative
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Primary amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 500 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |