| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:40 UTC |
|---|
| Update date | 2017-01-19 02:36:30 UTC |
|---|
| FoodComEx ID | PC000633 |
|---|
| FoodDB Record | FDB022505 |
|---|
| Chemical Information |
|---|
| Name | N-Acetylserotonin |
|---|
| Description | N-Acetylserotonin is an intermediate in the metabolic pathway of melatonin and indoleamine in the pineal gland of mammalians. Serotonin-N-acetyltransferase (SNAT), which regulates the rate of melatonin biosynthesis in the pineal gland, catalyzes the acetylation of 5HT to N-acetylserotonin (NAS). A methyl group from S-adenosylmethionine is transferred to NAS by hydroxyindole-O-methyltransferase (HIOMT), and finally NAS is converted to 5-methoxy-N-acetyltryptamine, or melatonin. In most mammalian species the content of NAS (and melatonin) in the pineal gland shows clear circadian changes with the highest level occurring during the dark period. This elevation of the contents of NAS (and melatonin) in the dark period is due to the increase of SNAT activity and the elevation of SNAT gene expression.
Experimental studies show that N-acetylserotonin possess free radical scavenging activity. Acute administration of irreversible and reversible selective MAO-A inhibitors and high doses (or chronic administration of low doses) of relatively selective MAO-B inhibitors (but not of highly selective MAO-B inhibitors) suppressed MAO-A activity and stimulated N-acetylation of pineal serotonin into N-acetylserotonin, the immediate precursor of melatonin. N-acetylserotonin increase after MAO-A inhibitors might mediate their antidepressive and antihypertensive effects. N-Acetylserotonin is the product of the O-demethylation of melatonin mediated by cytochrome P-450 isoforms: Cytochrome p450, subfamily IIc, polypeptide 19 (CYP2C19, a clinically important enzyme that metabolizes a wide variety of drugs), with a minor contribution from Cytochrome p450, subfamily I, polypeptide (2CYP1A2, involved in O-deethylation of phenacetin).
(PMID 15616152, 11103901, 10721079, 10591054) [HMDB]. N-Acetylserotonin is found in many foods, some of which are oregon yampah, barley, ostrich fern, and lovage. |
|---|
| CAS Number | 1210-83-9 |
|---|
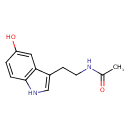
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 5-Hydroxy-N-acetyltryptamine | hmdb | | 5-Hydroxymelatonin | hmdb | | ASE | hmdb | | Desmethylmelatonin | HMDB | | N-(2-(5-Hydroxy-1H-indol-3-yl)ethyl)acetamide | ChEBI | | N-Acetyl-5-hydroxytryptamine | hmdb | | N-Acetylserotonin | hmdb | | O-Demethylmelatonin | HMDB |
|
|---|
| Chemical Formula | C12H14N2O2 |
|---|
| IUPAC name | N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]acetamide |
|---|
| InChI Identifier | InChI=1S/C12H14N2O2/c1-8(15)13-5-4-9-7-14-12-3-2-10(16)6-11(9)12/h2-3,6-7,14,16H,4-5H2,1H3,(H,13,15) |
|---|
| InChI Key | MVAWJSIDNICKHF-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CC(=O)NCCC1=CNC2=C1C=C(O)C=C2 |
|---|
| Average Molecular Weight | 218.2518 |
|---|
| Monoisotopic Molecular Weight | 218.105527702 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as hydroxyindoles. These are organic compounds containing an indole moiety that carries a hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Hydroxyindoles |
|---|
| Direct Parent | Hydroxyindoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxyindole
- 3-alkylindole
- Indole
- 1-hydroxy-2-unsubstituted benzenoid
- Substituted pyrrole
- Benzenoid
- Pyrrole
- Heteroaromatic compound
- Carboximidic acid
- Carboximidic acid derivative
- Azacycle
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Organic oxygen compound
- Organooxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 400 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 7506AH |
|---|
| Cayman Chemical | 14535 |
|---|
| MetaSci | HMDB0001238 |
|---|
| Toronto Research Chemicals | A179160 |
|---|
| Toronto Research Chemicals | HMDB0001238 |
|---|