| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:37 UTC |
|---|
| Update date | 2017-01-19 02:36:30 UTC |
|---|
| FoodComEx ID | PC000630 |
|---|
| FoodDB Record | FDB003293 |
|---|
| Chemical Information |
|---|
| Name | D-Lactic acid |
|---|
| Description | L-Lactic acid, also known as L-lactate or L-milchsaeure, belongs to the class of organic compounds known as alpha hydroxy acids and derivatives. These are organic compounds containing a carboxylic acid substituted with a hydroxyl group on the adjacent carbon. L-Lactic acid is an extremely weak basic (essentially neutral) compound (based on its pKa). Within humans, L-lactic acid participates in a number of enzymatic reactions. In particular, L-lactic acid can be biosynthesized from pyruvic acid through the action of the enzyme L-lactate dehydrogenase. In addition, L-lactic acid can be converted into L-lactic acid through the action of the enzyme monocarboxylate transporter. In humans, L-lactic acid is involved in the metabolic disorder called the glutaminolysis and cancer pathway. L-Lactic acid is an acidic and odorless tasting compound. Outside of the human body, L-Lactic acid is found, on average, in the highest concentration within beers and milk (cow). L-Lactic acid has also been detected, but not quantified in, several different foods, such as cow milks, cow milks, cow milks, cow milks, and port wines. This could make L-lactic acid a potential biomarker for the consumption of these foods. L-Lactic acid is a potentially toxic compound. An optically active form of lactic acid having (S)-configuration. |
|---|
| CAS Number | 10326-41-7 |
|---|
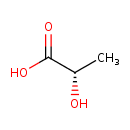
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (-)-Lactate | biospider | | (-)-Lactic acid | biospider | | (2R)-2-Hydroxypropanoic acid | biospider | | (D)-(-)-Lactic acid | biospider | | (R)-(-)-Lactate | biospider | | (R)-(-)-Lactic acid | biospider | | (R)-2-Hydroxypropanoate | biospider | | (R)-2-Hydroxypropanoic acid | biospider | | (R)-2-Hydroxypropionate | biospider | | (R)-2-Hydroxypropionic acid | biospider | | (R)-a-Hydroxypropionate | biospider | | (R)-a-Hydroxypropionic acid | biospider | | (R)-alpha-Hydroxypropionate | biospider | | (R)-alpha-Hydroxypropionic acid | biospider | | (R)-Lactate | biospider | | (R)-Lactic acid | biospider | | 2-Hydroxypropanoic acid, 9CI; D-form | db_source | | D-(-)-Lactate | biospider | | D-(-)-Lactic acid | biospider | | D-2-Hydroxypropanoate | biospider | | D-2-Hydroxypropanoic acid | biospider | | D-2-Hydroxypropionate | biospider | | D-2-Hydroxypropionic acid | biospider | | D-Lactate | biospider | | D-Lactic acid | biospider | | D-Milchsaeure | ChEBI | | delta-(-)-Lactate | HMDB | | delta-(-)-Lactic acid | HMDB | | delta-2-Hydroxypropanoate | HMDB | | delta-2-Hydroxypropanoic acid | HMDB | | delta-2-Hydroxypropionate | HMDB | | delta-2-Hydroxypropionic acid | HMDB | | delta-Lactate | HMDB | | delta-Lactic acid | HMDB | | DLA | HMDB | | L-(+)-Lactate | HMDB | | LACTIC ACID | ChEBI | | Propanoic acid, 2-hydroxy-, (2R)- | biospider | | Propanoic acid, 2-hydroxy-, (R)- | biospider | | Propel | HMDB |
|
|---|
| Chemical Formula | C3H6O3 |
|---|
| IUPAC name | (2S)-2-hydroxypropanoic acid |
|---|
| InChI Identifier | InChI=1S/C3H6O3/c1-2(4)3(5)6/h2,4H,1H3,(H,5,6)/t2-/m0/s1 |
|---|
| InChI Key | JVTAAEKCZFNVCJ-REOHCLBHSA-N |
|---|
| Isomeric SMILES | C[C@H](O)C(O)=O |
|---|
| Average Molecular Weight | 90.0779 |
|---|
| Monoisotopic Molecular Weight | 90.031694058 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alpha hydroxy acids and derivatives. These are organic compounds containing a carboxylic acid substituted with a hydroxyl group on the adjacent carbon. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Alpha hydroxy acids and derivatives |
|---|
| Direct Parent | Alpha hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-hydroxy acid
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 53° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 5 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | liquid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | X8654 |
|---|
| Toronto Research Chemicals | L113485 |
|---|