| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:32 UTC |
|---|
| Update date | 2017-01-19 02:36:30 UTC |
|---|
| FoodComEx ID | PC000620 |
|---|
| FoodDB Record | FDB022515 |
|---|
| Chemical Information |
|---|
| Name | Glucosamine 6-phosphate |
|---|
| Description | Glucosamine 6-phosphate is normally produced in endothelial cells via the de novo glucosamine synthesis by the enzyme fructose-6-phosphate amidotransferase and the modulation of this pathway by hyperglycemia and glutamine. glutamine-fructose-6-phosphate amidotransferase (GFAT) catalyzes the first committed step in the pathway for biosynthesis of hexosamines in mammals. A member of the N-terminal nucleophile class of amidotransferases, GFAT transfers the amino group from the L-glutamine amide to D-fructose 6-phosphate, producing glutamic acid and glucosamine 6-phosphate. As glucosamine inhibits endothelial nitric oxide synthesis it has important implications for impaired endothelium-dependent relaxation and vascular dysfunction in diabetes mellitus. (PMID 11270676, 11842094) [HMDB] |
|---|
| CAS Number | 3616-42-0 |
|---|
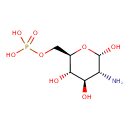
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 2-amino-2-Deoxy-6-O-ONO-a-D-glucopyranose | Generator | | 2-amino-2-Deoxy-6-O-ONO-alpha-D-glucopyranose | ChEBI | | 2-amino-2-Deoxy-6-O-ONO-α-D-glucopyranose | Generator | | 2-amino-2-Deoxy-D-glucose 6-ate | HMDB | | 2-Amino-2-deoxy-D-glucose 6-phosphate | hmdb | | 2-amino-2-Deoxyglucose 6-ate | HMDB | | 2-Amino-2-deoxyglucose 6-phosphate | hmdb | | 2-amino-D-Glucose-6-ate | HMDB | | 2-Amino-D-glucose-6-phosphate | hmdb | | a-D-Glucosamine 6-(dihydrogen ate) | Generator | | a-D-Glucosamine 6-(dihydrogen ic acid) | Generator | | alpha-D-Glucosamine 6-(dihydrogen ate) | ChEBI | | alpha-D-Glucosamine 6-(dihydrogen ic acid) | Generator | | D-Glucosamine 6-ate | HMDB | | D-Glucosamine 6-phosphate | hmdb | | D-Glucosamine ate | HMDB | | D-Glucosamine phosphate | hmdb | | D-Glucosamine-6-ate | HMDB | | D-Glucosamine-6-phosphate | hmdb | | Glucosamine 6 -ate | HMDB | | Glucosamine 6 -phosphate | hmdb | | GLUCOSAMINE 6-ATE | ChEBI | | GLUCOSAMINE 6-ic acid | Generator | | Glucosamine 6-Phosphate | hmdb | | Glucosamine 6-phosphic acid | hmdb | | Glucosamine-6-ate | HMDB | | glucosamine-6-P | hmdb | | glucosamine-6-phosphate | hmdb | | Glucose-6-orate | HMDB | | Glucose-6-oric acid | HMDB | | Glucose-6-phosphorate | hmdb | | Glucose-6-phosphoric acid | hmdb | | Oric acid mono-((2R,3S,4R,5R)-5-amino-2,3,4-trihydroxy-6-oxo-hexyl) ester | HMDB | | Phosphoric acid mono-((2R,3S,4R,5R)-5-amino-2,3,4-trihydroxy-6-oxo-hexyl) ester | hmdb | | α-D-glucosamine 6-(dihydrogen ate) | Generator | | α-D-glucosamine 6-(dihydrogen ic acid) | Generator |
|
|---|
| Chemical Formula | C6H14NO8P |
|---|
| IUPAC name | {[(2R,3S,4R,5R,6S)-5-amino-3,4,6-trihydroxyoxan-2-yl]methoxy}phosphonic acid |
|---|
| InChI Identifier | InChI=1S/C6H14NO8P/c7-3-5(9)4(8)2(15-6(3)10)1-14-16(11,12)13/h2-6,8-10H,1,7H2,(H2,11,12,13)/t2-,3-,4-,5-,6+/m1/s1 |
|---|
| InChI Key | XHMJOUIAFHJHBW-UKFBFLRUSA-N |
|---|
| Isomeric SMILES | N[C@H]1[C@@H](O)O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O |
|---|
| Average Molecular Weight | 259.151 |
|---|
| Monoisotopic Molecular Weight | 259.045702941 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as hexose phosphates. These are carbohydrate derivatives containing a hexose substituted by one or more phosphate groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Hexose phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hexose phosphate
- Monosaccharide phosphate
- Amino saccharide
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- Oxane
- Phosphoric acid ester
- Alkyl phosphate
- Hemiacetal
- 1,2-diol
- Secondary alcohol
- 1,2-aminoalcohol
- Organoheterocyclic compound
- Oxacycle
- Polyol
- Hydrocarbon derivative
- Organonitrogen compound
- Organopnictogen compound
- Primary aliphatic amine
- Organic nitrogen compound
- Organic oxide
- Amine
- Primary amine
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | - 2-amino-2-deoxy-D-glucopyranose 6-phosphate (CHEBI:15873 )
|
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 100 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | liquid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 6590AH |
|---|
| MetaSci | HMDB0001254 |
|---|
| Sigma-Aldrich | HMDB0001254 |
|---|
| Toronto Research Chemicals | G515050 |
|---|