| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:22 UTC |
|---|
| Update date | 2017-01-19 02:36:28 UTC |
|---|
| FoodComEx ID | PC000584 |
|---|
| FoodDB Record | FDB023111 |
|---|
| Chemical Information |
|---|
| Name | Biocytin |
|---|
| Description | Biocytin is a naturally occurring low molecular weight analog of biotin, and a primary source of this essential metabolite for mammals. Biotinidase acts as a hydrolase by cleaving biocytin and biotinyl-peptides, thereby liberating biotin for reutilization. Mammals cannot synthesize biotin and, therefore, derive the vitamin from dietary sources or from the endogenous turnover of the carboxylases. Free biotin can readily enter the biotin pool, whereas holocarboxylases or other biotin-containing proteins must first be degraded proteolytically to biocytin (biotinyl-e-lysine) or biotinyl-peptides. Biocytin is also an especially versatile marker for neuroanatomical investigations, shown that may have multiple applications, especially for labeling neurons. (PMID: 8930409, 1384763, 2479450) [HMDB] |
|---|
| CAS Number | 576-19-2 |
|---|
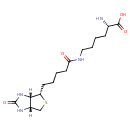
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (3AS-(3aalpha,4b,6aalpha))-N(6)-(5-(hexahydro-2-oxo-1H-thieno(3,4-D)imidazol-4-yl)-1-oxopentyl)-L-lysine | Generator | | (3AS-(3aalpha,4beta,6aalpha))-N(6)-(5-(hexahydro-2-oxo-1H-thieno(3,4-D)imidazol-4-yl)-1-oxopentyl)-L-lysine | ChEBI | | (3AS-(3aalpha,4β,6aalpha))-N(6)-(5-(hexahydro-2-oxo-1H-thieno(3,4-D)imidazol-4-yl)-1-oxopentyl)-L-lysine | Generator | | Biotinyl-L-lysine | hmdb | | epsilon-N-Biotinyl-L-lysine | hmdb | | epsilon-N-Biotinyllysine | ChEBI | | H-Lys(biotinyl)-OH | hmdb | | N-Biotinyl-L-lysine | ChEBI | | N-epsilon-Biotin-L-lysine | hmdb | | N(6)-D-Biotinyl-L-lysine | ChEBI | | N(epsilon)-Biotinyl-L-lysine | ChEBI | | N6-D-Biotinyl-L-lysine | hmdb | | N6-delta-Biotinyl-L-lysine | hmdb | | Ne-Biotynyl-L-lysine | hmdb |
|
|---|
| Chemical Formula | C16H28N4O4S |
|---|
| IUPAC name | (2S)-6-{5-[(3aS,4S,6aR)-2-oxo-hexahydro-1H-thieno[3,4-d]imidazol-4-yl]pentanamido}-2-aminohexanoic acid |
|---|
| InChI Identifier | InChI=1S/C16H28N4O4S/c17-10(15(22)23)5-3-4-8-18-13(21)7-2-1-6-12-14-11(9-25-12)19-16(24)20-14/h10-12,14H,1-9,17H2,(H,18,21)(H,22,23)(H2,19,20,24)/t10-,11-,12-,14-/m0/s1 |
|---|
| InChI Key | BAQMYDQNMFBZNA-MNXVOIDGSA-N |
|---|
| Isomeric SMILES | [H][C@]12CS[C@@H](CCCCC(=O)NCCCC[C@H](N)C(O)=O)[C@@]1([H])NC(=O)N2 |
|---|
| Average Molecular Weight | 372.483 |
|---|
| Monoisotopic Molecular Weight | 372.183126094 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - L-alpha-amino acid
- Imidazolyl carboxylic acid derivative
- Medium-chain fatty acid
- Heterocyclic fatty acid
- Fatty acid
- Fatty acyl
- Thiolane
- 2-imidazoline
- Amino acid
- Isourea
- Azacycle
- Carboximidic acid
- Carboximidic acid derivative
- Organoheterocyclic compound
- Dialkylthioether
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Carboxylic acid
- Thioether
- Monocarboxylic acid or derivatives
- Organic oxide
- Organonitrogen compound
- Primary aliphatic amine
- Organooxygen compound
- Organic oxygen compound
- Primary amine
- Organic nitrogen compound
- Organopnictogen compound
- Amine
- Carbonyl group
- Hydrocarbon derivative
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 20 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | Z5921 |
|---|
| Cayman Chemical | 16751 |
|---|
| Toronto Research Chemicals | B388100 |
|---|