| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:20 UTC |
|---|
| Update date | 2017-01-19 02:36:28 UTC |
|---|
| FoodComEx ID | PC000572 |
|---|
| FoodDB Record | FDB022754 |
|---|
| Chemical Information |
|---|
| Name | Medroxyprogesterone |
|---|
| Description | A synthetic progesterone (steroid hormone) involved in the female menstrual cycle, pregnancy (supports gestation) and embryogenesis of humans and other species. Progesterone belongs to a class of hormones called progestagens, and is the major naturally occurring human progestagen. -- Wikipedia
Progesterone's reproductive function serves to convert the endometrium to its secretory stage to prepare the uterus for implantation. If pregnancy does not occur, progesterone levels will decrease leading to menstruation in the human. Normal menstrual bleeding is a progesterone withdrawal bleeding. -- Wikipedia
During implantation and gestation, progesterone appears to decrease the maternal immune response to allow for the acceptance of the pregnancy. Progesterone decreases contractility of the uterine musculature. The fetus metabolizes placental progesterone in the production of adrenal mineralo and glucosteroids. A drop in progesterone levels is possibly one step that facilitates the onset of labor. In addition progesterone inhibits lactation during pregnancy. The fall in progesterone levels following delivery is one of the triggers for milk production. Progesterone has an effect upon vaginal epithelium and cervical mucus. -- Wikipedia
A synthetic progestational hormone used in veterinary practice as an estrus regulator. -- Pubchem [HMDB] |
|---|
| CAS Number | 520-85-4 |
|---|
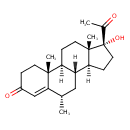
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 17-Hydroxy-6a-methyl-pregn-4-ene-3,20-dione | Generator | | 17-Hydroxy-6a-methylprogesterone | hmdb | | 17-Hydroxy-6alpha-methyl-pregn-4-ene-3,20-dione | ChEBI | | 17-Hydroxy-6alpha-methylprogesterone | hmdb | | 17-Hydroxy-6α-methyl-pregn-4-ene-3,20-dione | Generator | | 17-Hydroxy-6α-methylprogesterone | Generator | | 17a-Hydroxy-6a-methylprogesterone | Generator | | 17alpha-Hydroxy-6alpha-methylprogesterone | ChEBI | | 17α-hydroxy-6α-methylprogesterone | Generator | | 6a-Methyl-17a-hydroxyprogesterone | Generator | | 6a-Methyl-4-pregnen-17a-ol-3,20-dione | Generator | | 6alpha-Methyl-17alpha-hydroxyprogesterone | ChEBI | | 6alpha-Methyl-4-pregnen-17alpha-ol-3,20-dione | ChEBI | | 6α-methyl-17α-hydroxyprogesterone | Generator | | 6α-methyl-4-pregnen-17α-ol-3,20-dione | Generator | | Cycrin | hmdb | | Depo-Provera | hmdb | | Depo-Subq Provera 104 | hmdb | | Farlutal | hmdb | | Medrossiprogesterone | hmdb | | Medroxiprogesterona | hmdb | | Medroxiprogesteronum | hmdb | | Medroxyprogesteron | hmdb | | Medroxyprogesterone Acetate | hmdb | | Medroxyprogesteronum | hmdb | | Provera | hmdb |
|
|---|
| Chemical Formula | C22H32O3 |
|---|
| IUPAC name | (1S,2R,8S,10R,11S,14R,15S)-14-acetyl-14-hydroxy-2,8,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one |
|---|
| InChI Identifier | InChI=1S/C22H32O3/c1-13-11-16-17(20(3)8-5-15(24)12-19(13)20)6-9-21(4)18(16)7-10-22(21,25)14(2)23/h12-13,16-18,25H,5-11H2,1-4H3/t13-,16+,17-,18-,20+,21-,22-/m0/s1 |
|---|
| InChI Key | FRQMUZJSZHZSGN-HBNHAYAOSA-N |
|---|
| Isomeric SMILES | [H][C@@]12CC[C@](O)(C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@H](C)C2=CC(=O)CC[C@]12C |
|---|
| Average Molecular Weight | 344.4877 |
|---|
| Monoisotopic Molecular Weight | 344.23514489 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Pregnane steroids |
|---|
| Direct Parent | Gluco/mineralocorticoids, progestogins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 20-oxosteroid
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 17-hydroxysteroid
- Oxosteroid
- Hydroxysteroid
- Delta-4-steroid
- Cyclohexenone
- Alpha-hydroxy ketone
- Cyclic alcohol
- Tertiary alcohol
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Organooxygen compound
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 30 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 3808AH |
|---|
| MetaSci | HMDB0001939 |
|---|
| Toronto Research Chemicals | M203550 |
|---|
| Toronto Research Chemicals | HMDB0001939 |
|---|