| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:20 UTC |
|---|
| Update date | 2017-01-19 02:36:28 UTC |
|---|
| FoodComEx ID | PC000571 |
|---|
| FoodDB Record | FDB022753 |
|---|
| Chemical Information |
|---|
| Name | Lisinopril |
|---|
| Description | One of the Angiotensin-converting enzyme inhibitors (ACE inhibitors), orally active, that has been used in the treatment of hypertension and congestive heart failure. -- Pubchem; Lisinopril is a drug of the angiotensin converting enzyme (ACE) inhibitor class that is primarily used in treatment of hypertension, congestive heart failure and heart attacks. Historically, lisinopril was the third ACE inhibitor, after captopril and enalapril that was introduced into therapy in early 1990s . Lisinopril has a number of properties that distinguish it from other ACE inhibitors: it is hydrophilic, has long half life and tissue penetration and is not metabolized by the liver. -- Wikipedia; Lisinopril is solely excreted in urine in the unchanged form. Elimination of the drug depends on glomerular filtration and tubular excretion. Rate of lisinopril elimination decreases with old age and kidney or heart failure. There is a relation between creatinine and lisinopril clearance. With prolonged therapy dose reduction can be necessary to avoid accumulation. -- Wikipedia [HMDB] |
|---|
| CAS Number | 83915-83-7 |
|---|
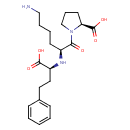
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (S)-1-(N(2)-(1-Carboxy-3-phenylpropyl)-L-lysyl)-L-proline | ChEBI | | [N2-[(S)-1-CARBOXY-3-phenylpropyl]-L-lysyl-L-proline | ChEBI | | Acerbon | hmdb | | Acercomp | hmdb | | Alapril | hmdb | | Carace | hmdb | | Cipral | hmdb | | Cipril | hmdb | | Coric | hmdb | | Inhibril | hmdb | | Inopril | hmdb | | Linopril | hmdb | | Linvas | hmdb | | Lipril | hmdb | | Lisinal | hmdb | | Lisinopril | hmdb | | Lisinopril anhydrous | hmdb | | Lisinoprilum | hmdb | | Lisipril | hmdb | | Lisoril | hmdb | | Lispril | hmdb | | Loril | hmdb | | LPR | hmdb | | Lysinopril | hmdb | | Noperten | hmdb | | Novatec | hmdb | | Presiten | hmdb | | Prinil | hmdb | | Prinivil | hmdb | | Sinopril | hmdb | | Sinopryl | hmdb | | Tensopril | hmdb | | Tensyn | hmdb | | Tersif | hmdb | | Vivatec | hmdb | | Zestril | hmdb |
|
|---|
| Chemical Formula | C21H31N3O5 |
|---|
| IUPAC name | (2S)-1-[(2S)-6-amino-2-{[(1S)-1-carboxy-3-phenylpropyl]amino}hexanoyl]pyrrolidine-2-carboxylic acid |
|---|
| InChI Identifier | InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 |
|---|
| InChI Key | RLAWWYSOJDYHDC-BZSNNMDCSA-N |
|---|
| Isomeric SMILES | NCCCC[C@H](N[C@@H](CCC1=CC=CC=C1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |
|---|
| Average Molecular Weight | 405.4879 |
|---|
| Monoisotopic Molecular Weight | 405.226371117 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- N-acyl-alpha-amino acid
- Proline or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-l-alpha-amino acid
- Alpha-amino acid amide
- Alpha-amino acid
- Alpha-amino acid or derivatives
- L-alpha-amino acid
- N-acylpyrrolidine
- Pyrrolidine carboxylic acid
- Pyrrolidine carboxylic acid or derivatives
- Aralkylamine
- Benzenoid
- Monocyclic benzene moiety
- Dicarboxylic acid or derivatives
- Tertiary carboxylic acid amide
- Pyrrolidine
- Amino acid or derivatives
- Carboxamide group
- Amino acid
- Organoheterocyclic compound
- Secondary amine
- Azacycle
- Carboxylic acid
- Secondary aliphatic amine
- Hydrocarbon derivative
- Primary aliphatic amine
- Organic oxide
- Organic nitrogen compound
- Organonitrogen compound
- Carbonyl group
- Organooxygen compound
- Primary amine
- Amine
- Organic oxygen compound
- Organopnictogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 10 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | H528 |
|---|