| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:19 UTC |
|---|
| Update date | 2017-01-19 02:36:28 UTC |
|---|
| FoodComEx ID | PC000564 |
|---|
| FoodDB Record | FDB000956 |
|---|
| Chemical Information |
|---|
| Name | Frusemide |
|---|
| Description | Potential contaminant in cow's milk arising from its use in dairy cattle for the treatment of physiological parturient edema
Additionally, furosemide is a noncompetitive subtype-specific blocker of GABA-A receptors. Furosemide has been reported to reversibly antagonize GABA-evoked currents of alpha6 beta2 gamma2 receptors at microM concentrations, but not alpha1 beta2 gamma2 receptors. During development, the alpha6 beta2 gamma2 receptor increases in expression in cerebellar granule neurons, corresponding to increased sensitivity to furosemide.; An antibiotic isolated from the fermentation broth of Fusidium coccineum. (From Merck Index, 11th ed) It acts by inhibiting translocation during protein synthesis.; Furosemide (INN) or frusemide (former BAN) is a loop diuretic used in the treatment of congestive heart failure and edema. It is most commonly marketed by Sanofi-Aventis under the brand name Lasix. It has also been used to prevent thoroughbred and standardbred race horses from bleeding through the nose during races.; Furosemide or frusemide is a loop diuretic used in the treatment of congestive heart failure and edema. It is most commonly marketed by Aventis Pharma under the brand name Lasix. It has also been used to prevent thoroughbred race horses from bleeding through the nose during races.; Furosemide, a sulfonamide-type loop diuretic structurally related to bumetanide, is used to manage hypertension and edema associated with congestive heart failure, cirrhosis, and renal disease, including the nephrotic syndrome. |
|---|
| CAS Number | 54-31-9 |
|---|
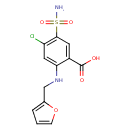
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 2-Furfurylamino-4-chloro-5-sulfamoylbenzoate | Generator | | 2-Furfurylamino-4-chloro-5-sulfamoylbenzoic acid | biospider | | 2-Furfurylamino-4-chloro-5-sulphamoylbenzoate | Generator | | 2-Furfurylamino-4-chloro-5-sulphamoylbenzoic acid | Generator | | 4-chloro-5-Sulfamoyl-N-furfuryl-anthranilate | Generator | | 4-Chloro-5-sulfamoyl-N-furfuryl-anthranilic acid | biospider | | 4-chloro-5-Sulphamoyl-N-furfuryl-anthranilate | Generator | | 4-chloro-5-Sulphamoyl-N-furfuryl-anthranilic acid | Generator | | 4-chloro-N-(2-Furylmethyl)-5-sulfamoylanthranilate | Generator | | 4-Chloro-N-(2-furylmethyl)-5-sulfamoylanthranilic acid | biospider | | 4-chloro-N-(2-Furylmethyl)-5-sulphamoylanthranilate | Generator | | 4-chloro-N-(2-Furylmethyl)-5-sulphamoylanthranilic acid | Generator | | 4-chloro-N-Furfuryl-5-sulfamoylanthranilate | Generator | | 4-Chloro-N-furfuryl-5-sulfamoylanthranilic acid | db_source | | 4-chloro-N-Furfuryl-5-sulphamoylanthranilate | Generator | | 4-chloro-N-Furfuryl-5-sulphamoylanthranilic acid | Generator | | 5-(Aminosulfonyl)-4-chloro-2-[2-(furanylmethyl)amino]benzoic acid, 9CI | db_source | | Acetic acid potassium salt | HMDB | | Aisemide | HMDB | | Aldalix | biospider | | Aldic | biospider | | Aluzine | biospider | | Anfuramaide | biospider | | Anthranilic acid, 4-chloro-N-furfuryl-5-sulfamoyl- | biospider | | Apo-frusemide | biospider | | Apo-furosemide | biospider | | Aquarid | biospider | | Aquasin | biospider | | Arasemide | biospider | | Beronald | HMDB | | Bio-furosemide | biospider | | Bioretic | biospider | | Cetasix | biospider | | Depix | biospider | | Desal | biospider | | Desdemin | HMDB | | Dirine | biospider | | Disal | HMDB | | Discoid | HMDB | | Disemide | biospider | | Diural | HMDB | | Diurapid | db_source | | Diuretic salt | HMDB | | Diurin | biospider | | Diurolasa | biospider | | Diusemide | biospider | | Diusil | biospider | | Diuzol | biospider | | Dom-furosemide | biospider | | Dranex | biospider | | Dryptal | db_source | | Durafurid | HMDB | | Edemid | biospider | | Edenol | biospider | | Eliur | biospider | | Endural | biospider | | Errolon | HMDB | | Eutensin | HMDB | | Farsix | biospider | | Fluidrol | biospider | | Fluss | biospider | | Franyl | biospider | | Froop | db_source | | Frumax | db_source | | Frumex | biospider | | Frumide | biospider | | Frumil | HMDB | | Frusedan | biospider | | Frusema | biospider | | Frusemid | biospider | | Frusemide | biospider | | Frusemide, BAN | db_source | | Frusemin | biospider | | Frusenex | biospider | | Frusetic | HMDB | | Frusid | db_source | | Frusol | db_source | | Fulsix | HMDB | | Fuluvamide | HMDB | | Fuluvamine | biospider | | Furanthril | biospider | | Furanthryl | biospider | | Furantril | biospider | | Furanturil | biospider | | Furesis | HMDB | | Furetic | biospider | | Furex | biospider | | Furfan | biospider | | Furix | biospider | | Furmid | biospider | | Furo-basan | biospider | | Furo-puren | HMDB | | Furobeta | biospider | | Furocot | biospider | | Furodiurol | biospider | | Furodrix | biospider | | Furomen | biospider | | Furomex | biospider | | Furomide m.d. | biospider | | Furorese | biospider | | Furosan | biospider | | Furose | biospider | | Furosedon | HMDB | | Furosemid | biospider | | Furosemida | biospider | | Furosemide "mita" | biospider | | Furosemide 40mg | biospider | | Furosemide special | biospider | | Furosemide(usan) | biospider | | Furosemidu | biospider | | Furosemidum | biospider | | Furosemix | biospider | | Furoside | biospider | | Furosifar | biospider | | Furosix | biospider | | Furoter | biospider | | Furovite | biospider | | Fursemid | biospider | | Fursemida | biospider | | Fursemide | db_source | | Fursol | biospider | | Fusid | biospider | | Fusidic acid | biospider | | Golan | biospider | | Hissuflux | biospider | | Hydrex | biospider | | Hydro | biospider | | hydro-Rapid | HMDB | | Hydroled | biospider | | Impugan | HMDB | | Jenafusid | biospider | | Katlex | HMDB | | Kofuzon | biospider | | Kolkin | biospider | | Kutrix | biospider | | Lasemid | biospider | | Lasex | biospider | | Lasiletten | biospider | | Lasilix | HMDB | | Lasix | db_source | | Lasix retard | biospider | | Lasix special | biospider | | Laxur | biospider | | Lazix | biospider | | Less diur | biospider | | Liside | biospider | | Logirene | biospider | | Lowpston | HMDB | | Lowpstron | biospider | | Luscek | biospider | | Macasirool | HMDB | | Marsemide | biospider | | Mirfat | HMDB | | MITA | biospider | | Moilarorin | biospider | | Nadis | biospider | | Nelsix | biospider | | Neo-renal | biospider | | Nephron | biospider | | Nicorol | HMDB | | Novosemide | biospider | | Octan draselny | HMDB | | Odemase | HMDB | | Odemex | biospider | | Oedemex | HMDB | | Osyrol | biospider | | PMS-furosemide | biospider | | Polysquall a | biospider | | Prefemin | biospider | | Profemin | HMDB | | Promedes | biospider | | Promide | biospider | | Protargen | biospider | | Puresis | biospider | | Radisemide | biospider | | Radonna | biospider | | Radouna | biospider | | Retep | biospider | | Rosemide | HMDB | | Rosis | biospider | | Rusyde | db_source | | Sal diureticum | HMDB | | Salinex | biospider | | Salix | biospider | | Salurex | biospider | | Salurid | biospider | | Seguril | biospider | | Selectofur | biospider | | Sigasalur | biospider | | Spirofur | biospider | | Synephron | biospider | | Tenkafruse | db_source | | Transit | biospider | | Trofurit | HMDB | | Uremide | biospider | | Uresix | biospider | | Urex | HMDB | | Urex-m | biospider | | Urian | biospider | | Uridon | biospider | | Uritol | biospider | | Urosemide | biospider | | Vesix | biospider | | Yidoli | biospider | | Zafimida | biospider |
|
|---|
| Chemical Formula | C12H11ClN2O5S |
|---|
| IUPAC name | 4-chloro-2-{[(furan-2-yl)methyl]amino}-5-sulfamoylbenzoic acid |
|---|
| InChI Identifier | InChI=1S/C12H11ClN2O5S/c13-9-5-10(15-6-7-2-1-3-20-7)8(12(16)17)4-11(9)21(14,18)19/h1-5,15H,6H2,(H,16,17)(H2,14,18,19) |
|---|
| InChI Key | ZZUFCTLCJUWOSV-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NS(=O)(=O)C1=C(Cl)C=C(NCC2=CC=CO2)C(=C1)C(O)=O |
|---|
| Average Molecular Weight | 330.744 |
|---|
| Monoisotopic Molecular Weight | 330.007719869 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as aminobenzenesulfonamides. These are organic compounds containing a benzenesulfonamide moiety with an amine group attached to the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzenesulfonamides |
|---|
| Direct Parent | Aminobenzenesulfonamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aminobenzenesulfonamide
- 4-halobenzoic acid or derivatives
- Aminobenzoic acid
- Aminobenzoic acid or derivatives
- Halobenzoic acid or derivatives
- 4-halobenzoic acid
- Halobenzoic acid
- Benzenesulfonyl group
- Benzoic acid
- Benzoic acid or derivatives
- Aniline or substituted anilines
- Phenylalkylamine
- Benzoyl
- Aralkylamine
- Secondary aliphatic/aromatic amine
- Halobenzene
- Chlorobenzene
- Organosulfonic acid amide
- Aryl chloride
- Aryl halide
- Furan
- Heteroaromatic compound
- Sulfonyl
- Organosulfonic acid or derivatives
- Organic sulfonic acid or derivatives
- Aminosulfonyl compound
- Vinylogous amide
- Amino acid or derivatives
- Amino acid
- Oxacycle
- Organoheterocyclic compound
- Secondary amine
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organopnictogen compound
- Organosulfur compound
- Organic oxygen compound
- Organic nitrogen compound
- Amine
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | 2.03 | SANGSTER (1993) |
|---|
| Experimental Water Solubility | 0.0731 mg/mL at 30 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | Mp 206° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 20 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | H788 |
|---|
| AKSci | J10011 |
|---|
| AKSci | J40149 |
|---|
| AKSci | J93989 |
|---|
| Cayman Chemical | 17273 |
|---|
| MetaSci | HMDB0001933 |
|---|
| Sigma-Aldrich | HMDB0001933 |
|---|
| Toronto Research Chemicals | F865000 |
|---|
| Toronto Research Chemicals | KIT1230 |
|---|