| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:18 UTC |
|---|
| Update date | 2017-01-19 02:36:28 UTC |
|---|
| FoodComEx ID | PC000559 |
|---|
| FoodDB Record | FDB022745 |
|---|
| Chemical Information |
|---|
| Name | Hydrochlorothiazide |
|---|
| Description | A thiazide diuretic often considered the prototypical member of this class. It reduces the reabsorption of electrolytes from the renal tubules. This results in increased excretion of water and electrolytes, including sodium, potassium, chloride, and magnesium. It has been used in the treatment of several disorders including edema, hypertension, diabetes insipidus, and hypoparathyroidism. -- Pubchem
Hydrochlorothiazide (Apo-Hydro, Aquazide H, Microzide, Oretic), sometimes abbreviated HCT, HCTZ, or HZT is a popular diuretic drug that acts by inhibiting the kidney's ability to retain water. This reduces the volume of the blood, decreasing peripheral vascular resistance. Chlorothiazide, a carbonic anhydrase inhibitor. --Wikipedia [HMDB] |
|---|
| CAS Number | 58-93-5 |
|---|
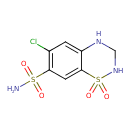
| Structure | |
|---|
| Synonyms | |
|---|
| Chemical Formula | C7H8ClN3O4S2 |
|---|
| IUPAC name | 6-chloro-1,1-dioxo-3,4-dihydro-2H-1lambda6,2,4-benzothiadiazine-7-sulfonamide |
|---|
| InChI Identifier | InChI=1S/C7H8ClN3O4S2/c8-4-1-5-7(2-6(4)16(9,12)13)17(14,15)11-3-10-5/h1-2,10-11H,3H2,(H2,9,12,13) |
|---|
| InChI Key | JZUFKLXOESDKRF-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NS(=O)(=O)C1=C(Cl)C=C2NCNS(=O)(=O)C2=C1 |
|---|
| Average Molecular Weight | 297.739 |
|---|
| Monoisotopic Molecular Weight | 296.964474846 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as 1,2,4-benzothiadiazine-1,1-dioxides. These are aromatic heterocyclic compounds containing a 1,2,4-benzothiadiazine ring system with two S=O bonds at the 1-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Thiadiazines |
|---|
| Sub Class | Benzothiadiazines |
|---|
| Direct Parent | 1,2,4-benzothiadiazine-1,1-dioxides |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2,4-benzothiadiazine-1,1-dioxide
- Secondary aliphatic/aromatic amine
- Aryl chloride
- Aryl halide
- Organosulfonic acid amide
- Benzenoid
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Aminosulfonyl compound
- Sulfonyl
- Secondary amine
- Azacycle
- Organic oxide
- Amine
- Organopnictogen compound
- Organosulfur compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
|
| Foods of Origin |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | C825 |
|---|
| AKSci | J10079 |
|---|
| AKSci | J92182 |
|---|
| Cayman Chemical | 21304 |
|---|
| Glentham | GE0436 |
|---|
| MetaSci | HMDB0001928 |
|---|
| Sigma-Aldrich | HMDB0001928 |
|---|
| Toronto Research Chemicals | H714560 |
|---|