| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:18 UTC |
|---|
| Update date | 2017-01-19 02:36:27 UTC |
|---|
| FoodComEx ID | PC000556 |
|---|
| FoodDB Record | FDB022741 |
|---|
| Chemical Information |
|---|
| Name | Naproxen |
|---|
| Description | Naproxen (INN) is a non-steroidal anti-inflammatory drug (NSAID) commonly used for the reduction of mild to moderate pain, fever, inflammation and stiffness caused by conditions such as osteoarthritis, rheumatoid arthritis, psoriatic arthritis, gout, ankylosing spondylitis, injury (like fractures), menstrual cramps, tendonitis, bursitis, and the treatment of primary dysmenorrhea. Naproxen and naproxen sodium are marketed under various trade names including: Aleve, Anaprox, Naprogesic, Naprosyn, Naprelan; Naproxen is a non-steroidal anti-inflammatory drug (NSAID) commonly used for the reduction of mild to moderate pain, fever, inflammation and stiffness caused by conditions such as osteoarthritis, rheumatoid arthritis, psoriatic arthritis, gout, ankylosing spondylitis, injury (like fractures), menstrual cramps, tendonitis, bursitis, and the treatment of primary dysmenorrhea. Naproxen and naproxen sodium are marketed under various trade names including: Aleve, Anaprox, Naprogesic, Naprosyn, Naprelan.
Naproxen was first marketed as the prescription drug Naprosyn in 1976 and naproxen sodium was first marketed under the trade name Anaprox in 1980. It remains a prescription-only drug in much of the world. The U.S. Food and Drug Administration (FDA) approved the use of naproxen sodium as an over-the-counter (OTC) drug in 1991, where OTC preparations are sold under the trade name Aleve. In Australia, small packets of lower-strength preparations of naproxen sodium are Schedule 2 Pharmacy Medicines; Naproxen is a member of the 2-arylpropionic acid (profen) family of NSAIDs. It is an odorless, white to off-white crystalline substance. It is lipid-soluble, practically insoluble in water with a low pH (below pH 4), while freely soluble in water at 6 pH and above. Naproxen has a melting point of 153 degree centigrade. [HMDB] |
|---|
| CAS Number | 22204-53-1 |
|---|
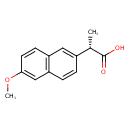
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (+)-(S)-6-Methoxy-a-methyl-2-naphthaleneacetate | Generator | | (+)-(S)-6-Methoxy-a-methyl-2-naphthaleneacetic acid | Generator | | (+)-(S)-6-Methoxy-alpha-methyl-2-naphthaleneacetate | Generator | | (+)-(S)-6-Methoxy-alpha-methyl-2-naphthaleneacetic acid | ChEBI | | (+)-(S)-6-Methoxy-α-methyl-2-naphthaleneacetate | Generator | | (+)-(S)-6-Methoxy-α-methyl-2-naphthaleneacetic acid | Generator | | (+)-(S)-Naproxen | hmdb | | (+)-2-(6-Methoxy-2-naphthyl)propionate | Generator | | (+)-2-(6-Methoxy-2-naphthyl)propionic acid | ChEBI | | (+)-2-(Methoxy-2-naphthyl)-propionate | Generator | | (+)-2-(Methoxy-2-naphthyl)-propionic acid | ChEBI | | (+)-2-(Methoxy-2-naphthyl)-propionsaeure | ChEBI | | (+)-Naproxen | hmdb | | (S)-(+)-2-(6-Methoxy-2-naphthyl)propionate | Generator | | (S)-(+)-2-(6-Methoxy-2-naphthyl)propionic acid | ChEBI | | (S)-(+)-Naproxen | ChEBI | | (S)-2-(6-Methoxy-2-naphthyl)propanoate | Generator | | (S)-2-(6-Methoxy-2-naphthyl)propanoic acid | ChEBI | | (S)-2-(6-Methoxy-2-naphthyl)propionate | Generator | | (S)-2-(6-Methoxy-2-naphthyl)propionic acid | ChEBI | | (S)-6-Methoxy-a-methyl-2-naphthaleneacetate | Generator | | (S)-6-Methoxy-a-methyl-2-naphthaleneacetic acid | Generator | | (S)-6-Methoxy-alpha-methyl-2-naphthaleneacetate | Generator | | (S)-6-Methoxy-alpha-methyl-2-naphthaleneacetic acid | ChEBI | | (S)-6-Methoxy-α-methyl-2-naphthaleneacetate | Generator | | (S)-6-Methoxy-α-methyl-2-naphthaleneacetic acid | Generator | | (S)-Naproxen | hmdb | | 2-(6-Methoxy-2-naphthyl)propionic acid | hmdb | | Acusprain | hmdb | | Anexopen | hmdb | | Apronax | hmdb | | Artagen | hmdb | | Arthrisil | hmdb | | Artrixen | hmdb | | Artroxen | hmdb | | Atiflan | hmdb | | Axer | hmdb | | Bipronyl | hmdb | | Calosen | hmdb | | Clinosyn | hmdb | | Congex | hmdb | | d-Naproxen | hmdb | | Danaprox | hmdb | | Daprox | hmdb | | Diocodal | hmdb | | DL Naproxen | hmdb | | DL-Naproxen | hmdb | | Duk | hmdb | | Dysmenalgit | hmdb | | Dysmenalgit N | hmdb | | Ec-Naprosyn | hmdb | | Equiproxen | hmdb | | Flexipen | hmdb | | Floginax | hmdb | | Fuxen | hmdb | | Genoxen | hmdb | | Lefaine | hmdb | | Leniartil | hmdb | | Nafasol | hmdb | | Naixan | hmdb | | Nalyxan | hmdb | | Napflam | hmdb | | Napmel | hmdb | | Naposin | hmdb | | Naprosyne | hmdb | | Naproxen | hmdb | | Naproxen Sodium | hmdb | | Naproxene | hmdb | | Naproxeno | hmdb | | Naproxenum | hmdb | | Novonaprox | hmdb | | Nycopren | hmdb | | Opipramol | hmdb |
|
|---|
| Chemical Formula | C14H14O3 |
|---|
| IUPAC name | (2S)-2-(6-methoxynaphthalen-2-yl)propanoic acid |
|---|
| InChI Identifier | InChI=1S/C14H14O3/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10/h3-9H,1-2H3,(H,15,16)/t9-/m0/s1 |
|---|
| InChI Key | CMWTZPSULFXXJA-VIFPVBQESA-N |
|---|
| Isomeric SMILES | COC1=CC2=C(C=C1)C=C(C=C2)[C@H](C)C(O)=O |
|---|
| Average Molecular Weight | 230.2592 |
|---|
| Monoisotopic Molecular Weight | 230.094294314 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as naphthalenes. Naphthalenes are compounds containing a naphthalene moiety, which consists of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Naphthalenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthalenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthalene
- Anisole
- Alkyl aryl ether
- Monocarboxylic acid or derivatives
- Ether
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | J10145 |
|---|
| AKSci | J92184 |
|---|
| AKSci | W4580 |
|---|
| Cayman Chemical | 70290 |
|---|
| Toronto Research Chemicals | N377520 |
|---|