| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:18 UTC |
|---|
| Update date | 2017-01-19 02:36:27 UTC |
|---|
| FoodComEx ID | PC000555 |
|---|
| FoodDB Record | FDB022740 |
|---|
| Chemical Information |
|---|
| Name | Clotrimazole |
|---|
| Description | Clotrimazole is an imidazole derivative with a broad spectrum of antimycotic activity. It inhibits biosynthesis of the sterol ergostol, an important component of fungal cell membranes. Its action leads to increased membrane permeability and apparent disruption of enzyme systems bound to the membrane. -- Pubchem; There is the potential for drug interactions with Clotrimazole if taken orally, as it is a potent, specific inhibitor of cytochrome P450 oxidase enzymes and so may alter the metabolism of other drugs.; Clotrimazole is an antifungal medication commonly used in the treatment of fungal infections of both humans and animals such as vaginal yeast infections and ringworm. -- Wikipedia; An imidazole derivative with a broad spectrum of antimycotic activity. It inhibits biosynthesis of the sterol ergostol, an important component of fungal cell membranes. Its action leads to increased membrane permeability and apparent disruption of enzyme systems bound to the membrane.; Clotrimazole is a potent, specific inhibitor of cytochrome P450 oxidase enzymes. Hence, it may alter the metabolism of other drugs particularly if taken orally. -- Wikipedia; Clotrimazole is an antifungal medication commonly used in the treatment of fungal infections of both humans and animals such as vaginal yeast infections and ringworm. It also used to treat athlete's foot and jock itch. [HMDB] |
|---|
| CAS Number | 23593-75-1 |
|---|
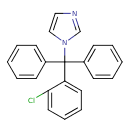
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (Chlorotrityl)imidazole | hmdb | | 1-((2-Chlorophenyl)diphenylmethyl)-1H-imidazole | ChEBI | | 1-(a-(2-Chlorophenyl)benzhydryl)imidazole | Generator | | 1-(alpha-(2-Chlorophenyl)benzhydryl)imidazole | ChEBI | | 1-(O-chloro-a,a-Diphenylbenzyl)imidazole | Generator | | 1-(O-chloro-alpha,alpha-Diphenylbenzyl)imidazole | ChEBI | | 1-(O-chloro-α,α-diphenylbenzyl)imidazole | Generator | | 1-(o-Chlorotrityl)imidazole | hmdb | | 1-(α-(2-chlorophenyl)benzhydryl)imidazole | Generator | | Canesten | hmdb | | Canesten 1-Day Cream Combi-Pak | hmdb | | Canesten 1-Day Therapy | hmdb | | Canesten 3-Day Therapy | hmdb | | Canesten 6-Day Therapy | hmdb | | Canesten Combi-Pak 1-Day Therapy | hmdb | | Canesten Combi-Pak 3-Day Therapy | hmdb | | Canesten Cream | hmdb | | Canesten Solution | hmdb | | Canestine | hmdb | | Chlotrimazole | hmdb | | Clotrimaderm | hmdb | | Clotrimaderm Cream | hmdb | | Clotrimazol | hmdb | | Clotrimazole | hmdb | | Clotrimazolum | hmdb | | Desamix F | hmdb | | Empecid | hmdb | | Fem Care | hmdb | | FemCare | hmdb | | Gyne lotrimin | hmdb | | Gyne-lotrimin | hmdb | | Gyne-Lotrimin 3 | hmdb | | Gyne-Lotrimin 3 Combination Pack | hmdb | | Gyne-Lotrimin Combination Pack | hmdb | | Gyne-Lotrimin3 | hmdb | | Gyne-Lotrimin3 Combination Pack | hmdb | | Gynix | hmdb | | Lopac-C-6019 | hmdb | | Lotrimax | hmdb | | Lotrimin | hmdb | | Lotrimin AF | hmdb | | Lotrimin AF Cream | hmdb | | Lotrimin AF Jock-Itch Cream | hmdb | | Lotrimin AF Lotion | hmdb | | Lotrimin AF Solution | hmdb | | Lotrimin Cream | hmdb | | Lotrimin Lotion | hmdb | | Lotrimin Solution | hmdb | | Lotrisone | hmdb | | Mono-baycuten | hmdb | | Monobaycuten | hmdb | | Mycelax | hmdb | | Mycelex | hmdb | | Mycelex 7 | hmdb | | Mycelex Cream | hmdb | | Mycelex G | hmdb | | Mycelex OTC | hmdb | | Mycelex Solution | hmdb | | Mycelex Troches | hmdb | | Mycelex Twin Pack | hmdb | | Mycelex-7 | hmdb | | Mycelex-7 Combination Pack | hmdb | | Mycelex-G | hmdb | | Mycelex: MycosporinRimazole | hmdb | | Myclo Cream | hmdb | | Myclo Solution | hmdb | | Myclo Spray Solution | hmdb | | Myclo-Gyne | hmdb | | Mycosporin | hmdb | | Mykosporin | hmdb | | Neo-Zol Cream | hmdb | | Otomax | hmdb | | Pedisafe | hmdb | | Prestwick_120 | hmdb | | Rimazole | hmdb | | Tibatin | hmdb | | Trimysten | hmdb | | Trivagizole 3 | hmdb | | Veltrim | hmdb |
|
|---|
| Chemical Formula | C22H17ClN2 |
|---|
| IUPAC name | 1-[(2-chlorophenyl)diphenylmethyl]-1H-imidazole |
|---|
| InChI Identifier | InChI=1S/C22H17ClN2/c23-21-14-8-7-13-20(21)22(25-16-15-24-17-25,18-9-3-1-4-10-18)19-11-5-2-6-12-19/h1-17H |
|---|
| InChI Key | VNFPBHJOKIVQEB-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | ClC1=CC=CC=C1C(N1C=CN=C1)(C1=CC=CC=C1)C1=CC=CC=C1 |
|---|
| Average Molecular Weight | 344.837 |
|---|
| Monoisotopic Molecular Weight | 344.108026261 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as triphenyl compounds. These are aromatic compounds containing a triphenyl moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Triphenyl compounds |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Triphenyl compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triphenyl compound
- Chlorobenzene
- Halobenzene
- Aryl chloride
- Aryl halide
- Monocyclic benzene moiety
- N-substituted imidazole
- Azole
- Heteroaromatic compound
- Imidazole
- Azacycle
- Organoheterocyclic compound
- Organonitrogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organochloride
- Organohalogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | J10369 |
|---|
| AKSci | N713 |
|---|
| Cayman Chemical | 15278 |
|---|
| Glentham | GA8137 |
|---|
| Toronto Research Chemicals | C587400 |
|---|