| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:18 UTC |
|---|
| Update date | 2017-01-19 02:36:27 UTC |
|---|
| FoodComEx ID | PC000548 |
|---|
| FoodDB Record | FDB004716 |
|---|
| Chemical Information |
|---|
| Name | Cotinine |
|---|
| Description | Cotinine has an in vivo half life of approximately 20 hours, and is typically detectable for several days to up to one week after the use of tobacco. The level of cotinine in the blood is proportionate to the amount of exposure to tobacco smoke, so it is a valuable indicator of tobacco smoke exposure, including secondary (passive) smoke. People who smoke menthol cigarettes may retain cotinine in the blood for a longer period because menthol can compete with cotinine enzymatic metabolism. Genetic encoding of liver enzymes may also play a role, as African Americans routinely register higher blood cotinine levels than Caucasians. Several variable factors, such as menthol cigarette preference and puff size, suggest that the explanation for this difference may be more complex than gender or race.[citation needed]; Cotinine is a metabolite of nicotine. The word 'cotinine' is an anagram of 'nicotine'. It is used to measure the grade of tobacco smoking, but might also improve mental function.; Quantitatively, the most important metabolite of nicotine in most mammalian species is cotinine. In humans, about 70 to 80% of nicotine is converted to cotinine. This transformation involves two steps. The first is mediated by a cytochrome P450 system (mainly CYP2A6 and CYP2B6) to produce nicotine iminium ion. The second step is catalyzed by aldehyde oxidase (AOX). A number of cotinine metabolites have also been structurally characterized. Indeed, it appears that most of the reported urinary metabolites of nicotine are derived from cotinine. Cotinine is found in many foods, some of which are ceylon cinnamon, arrowhead, mountain yam, and rambutan. |
|---|
| CAS Number | 486-56-6 |
|---|
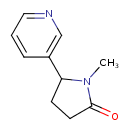
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (-)-cotinine | biospider | | (s)-(-)-cotinine | biospider | | (S)-1-Methyl-5-(3-pyridinyl)-2-pyrrolidinone | biospider | | (s)-cotinine | biospider | | 1-Methyl-5-(3-pyridinyl)-2-pyrrolidinone | biospider | | 1-methyl-5-pyridin-3-ylpyrrolidin-2-one | biospider | | 2-Pyrrolidinone, 1-methyl-5-(3-pyridinyl)- | biospider | | 2-Pyrrolidinone, 1-methyl-5-(3-pyridinyl)-, (5S)- | biospider | | 2-Pyrrolidinone, 1-methyl-5-(3-pyridinyl)-, (S)- | biospider | | 2-Pyrrolidinone, 1-methyl-5-(3-pyridinyl)-, (S)- (9CI) | biospider | | Cotinina | biospider | | Cotinine | biospider | | Cotinine [inn] | biospider | | Cotininum | biospider | | S-(-)-cotinine | biospider | | Scotine | biospider |
|
|---|
| Chemical Formula | C10H12N2O |
|---|
| IUPAC name | 1-methyl-5-(pyridin-3-yl)pyrrolidin-2-one |
|---|
| InChI Identifier | InChI=1S/C10H12N2O/c1-12-9(4-5-10(12)13)8-3-2-6-11-7-8/h2-3,6-7,9H,4-5H2,1H3 |
|---|
| InChI Key | UIKROCXWUNQSPJ-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CN1C(CCC1=O)C1=CN=CC=C1 |
|---|
| Average Molecular Weight | 176.2151 |
|---|
| Monoisotopic Molecular Weight | 176.094963016 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as pyrrolidinylpyridines. Pyrrolidinylpyridines are compounds containing a pyrrolidinylpyridine ring system, which consists of a pyrrolidine ring linked to a pyridine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pyrrolidinylpyridines |
|---|
| Direct Parent | Pyrrolidinylpyridines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrrolidinylpyridine
- Alkaloid or derivatives
- Pyrrolidone
- 2-pyrrolidone
- N-alkylpyrrolidine
- Pyrrolidine
- Tertiary carboxylic acid amide
- Heteroaromatic compound
- Lactam
- Carboxamide group
- Carboxylic acid derivative
- Azacycle
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | 0.07 | LI,NY & GORROD,JW (1992) |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | 41 oC | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 40 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 4205AE |
|---|
| Cayman Chemical | 15314 |
|---|
| Cayman Chemical | HMDB0001046 |
|---|
| MetaSci | HMDB0001046 |
|---|
| Toronto Research Chemicals | C725000 |
|---|
| Toronto Research Chemicals | KIT0540 |
|---|