| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:16 UTC |
|---|
| Update date | 2017-01-19 02:36:27 UTC |
|---|
| FoodComEx ID | PC000542 |
|---|
| FoodDB Record | FDB021901 |
|---|
| Chemical Information |
|---|
| Name | Inosinic acid |
|---|
| Description | Inosinic acid, also known as 5'-inosinate or 5'-IMP, belongs to the class of organic compounds known as purine ribonucleoside monophosphates. These are nucleotides consisting of a purine base linked to a ribose to which one monophosphate group is attached. Inosinic acid is an extremely weak basic (essentially neutral) compound (based on its pKa). Inosinic acid exists in all living species, ranging from bacteria to humans. Within humans, inosinic acid participates in a number of enzymatic reactions. In particular, inosinic acid can be converted into phosphoribosyl formamidocarboxamide through the action of the enzyme bifunctional purine biosynthesis protein purh. In addition, inosinic acid can be converted into xanthylic acid through its interaction with the enzyme inosine-5'-monophosphate dehydrogenase 1. In humans, inosinic acid is involved in the metabolic disorder called the gout or kelley-seegmiller syndrome pathway. Inosinic acid is an odorless tasting compound. Outside of the human body, Inosinic acid has been detected, but not quantified in, several different foods, such as hickory nuts, turnips, pepper (spice), mountain yams, and muskmelons. This could make inosinic acid a potential biomarker for the consumption of these foods. A purine ribonucleoside 5'-monophosphate having hypoxanthine as the nucleobase. |
|---|
| CAS Number | 131-99-7 |
|---|
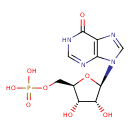
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 2'-Inosine-5'-monoate | ChEBI | | 2'-Inosine-5'-monoic acid | Generator | | 5'-IMP | hmdb | | 5'-Inosinate | hmdb | | 5'-Inosine monoate | ChEBI | | 5'-Inosine monoic acid | Generator | | 5'-Inosine monophosphate | hmdb | | 5'-Inosinic acid | hmdb | | Hypoxanthosine 5'-monoate | ChEBI | | Hypoxanthosine 5'-monoic acid | Generator | | IMP | hmdb | | Inosinate | Generator | | Inosine 5'-ate | ChEBI | | Inosine 5'-ic acid | Generator | | Inosine 5'-monoate | ChEBI | | Inosine 5'-monoic acid | Generator | | Inosine 5'-monophosphate | hmdb | | Inosine 5'-phosphate | hmdb | | Inosine monoate | ChEBI | | Inosine monoic acid | Generator | | Inosine Monophosphate | hmdb | | Inosine-5'-monoate | HMDB | | Inosine-5'-monophosphate | hmdb | | Ribosylhypoxanthine monoate | ChEBI | | Ribosylhypoxanthine monoic acid | Generator |
|
|---|
| Chemical Formula | C10H13N4O8P |
|---|
| IUPAC name | {[(2R,3S,4R,5R)-3,4-dihydroxy-5-(6-oxo-6,9-dihydro-1H-purin-9-yl)oxolan-2-yl]methoxy}phosphonic acid |
|---|
| InChI Identifier | InChI=1S/C10H13N4O8P/c15-6-4(1-21-23(18,19)20)22-10(7(6)16)14-3-13-5-8(14)11-2-12-9(5)17/h2-4,6-7,10,15-16H,1H2,(H,11,12,17)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 |
|---|
| InChI Key | GRSZFWQUAKGDAV-KQYNXXCUSA-N |
|---|
| Isomeric SMILES | O[C@@H]1[C@@H](COP(O)(O)=O)O[C@H]([C@@H]1O)N1C=NC2=C1N=CNC2=O |
|---|
| Average Molecular Weight | 348.206 |
|---|
| Monoisotopic Molecular Weight | 348.047099924 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as purine ribonucleoside monophosphates. These are nucleotides consisting of a purine base linked to a ribose to which one monophosphate group is attached. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Purine nucleotides |
|---|
| Sub Class | Purine ribonucleotides |
|---|
| Direct Parent | Purine ribonucleoside monophosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Purine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-oxopurine
- Hypoxanthine
- Monosaccharide phosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Pyrimidone
- Monoalkyl phosphate
- Alkyl phosphate
- Pyrimidine
- Phosphoric acid ester
- Organic phosphoric acid derivative
- N-substituted imidazole
- Monosaccharide
- Tetrahydrofuran
- Vinylogous amide
- Azole
- Imidazole
- Heteroaromatic compound
- Secondary alcohol
- 1,2-diol
- Lactam
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Alcohol
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 7272AH |
|---|