| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:14 UTC |
|---|
| Update date | 2017-01-19 02:36:27 UTC |
|---|
| FoodComEx ID | PC000534 |
|---|
| FoodDB Record | FDB012833 |
|---|
| Chemical Information |
|---|
| Name | Cytidine 5'-triphosphate |
|---|
| Description | Found in cheese and liver
CTP is a high-energy molecule equal to ATP, but its role in the organism is more specific than that of ATP. CTP is used as the source of energy, and as a coenzyme in metabolic reactions like the synthesis of glycerophospholipids and glycosylation of proteins.; CTP is a substrate in the synthesis of RNA.; Cytidine 5'-(tetrahydrogen triphosphate) or CTP is a cytosine nucleotide containing three phosphate groups esterified to a ribose moiety at the 5' position. CTP is integral to the synthesis or mRNA, rRNA and tRNA through RNA polymerases. Cytidine triphosphate (CTP) is also critical to the synthesis of phosphatidylcholine via the enzyme CTP: phosphocholine cytidyltransferase. This reaction is the rate-limiting step in the synthesis of phosphatidylcholine.; Cytidine triphosphate is a pyrimidine nucleotide. |
|---|
| CAS Number | 65-47-4 |
|---|
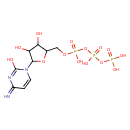
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1wvc | biospider | | 5'-(Tetrahydrogen triate) cytidine | HMDB | | 5'-(tetrahydrogen triphosphate) cytidine | biospider | | 5'-CTP | biospider | | CTP | biospider | | Cytidine 3'-triate | HMDB | | Cytidine 3'-triphosphate | biospider | | Cytidine 5-prime-triate | HMDB | | Cytidine 5-Prime-Triphosphate | biospider | | Cytidine 5'-(tetrahydrogen triate) | HMDB | | cytidine 5'-(tetrahydrogen triphosphate) | biospider | | Cytidine 5'-(tetrahydrogen triphosphate) (8CI,9CI) | biospider | | Cytidine 5'-(tetrahydrogen triphosphate), 9CI | db_source | | Cytidine 5'-triate | ChEBI | | Cytidine 5'-triic acid | Generator | | Cytidine 5'-trioric acid | HMDB | | Cytidine 5'-triphosphoric acid | db_source | | Cytidine mono | biospider | | Cytidine mono(tetrahydrogen triate) (ester) | HMDB | | Cytidine mono(tetrahydrogen triphosphate) (ester) | biospider | | Cytidine triate | ChEBI | | Cytidine triic acid | Generator | | Cytidine triphosphate | biospider | | Cytidine triphosphic acid | biospider | | CYTIDINE-5'-triATE | ChEBI | | CYTIDINE-5'-triic acid | Generator | | cytidine-5'-triphosphate | biospider | | Cytidine, mono(tetrahydrogen triphosphate) (ester) | biospider | | Deoxycytosine triate | HMDB | | Deoxycytosine triphosphate | biospider | | H4ctp | biospider |
|
|---|
| Chemical Formula | C9H16N3O14P3 |
|---|
| IUPAC name | ({[({[3,4-dihydroxy-5-(2-hydroxy-4-imino-1,4-dihydropyrimidin-1-yl)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)phosphonic acid |
|---|
| InChI Identifier | InChI=1S/C9H16N3O14P3/c10-5-1-2-12(9(15)11-5)8-7(14)6(13)4(24-8)3-23-28(19,20)26-29(21,22)25-27(16,17)18/h1-2,4,6-8,13-14H,3H2,(H,19,20)(H,21,22)(H2,10,11,15)(H2,16,17,18) |
|---|
| InChI Key | PCDQPRRSZKQHHS-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NC1=NC(=O)N(C=C1)C1OC(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)C(O)C1O |
|---|
| Average Molecular Weight | 483.1563 |
|---|
| Monoisotopic Molecular Weight | 482.984511771 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as pyrimidine ribonucleoside triphosphates. These are pyrimidine ribobucleotides with triphosphate group linked to the ribose moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Pyrimidine nucleotides |
|---|
| Sub Class | Pyrimidine ribonucleotides |
|---|
| Direct Parent | Pyrimidine ribonucleoside triphosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidine ribonucleoside triphosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Pentose monosaccharide
- Aminopyrimidine
- Pyrimidone
- Monoalkyl phosphate
- Hydropyrimidine
- Monosaccharide
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Alkyl phosphate
- Imidolactam
- Tetrahydrofuran
- Heteroaromatic compound
- Secondary alcohol
- 1,2-diol
- Oxacycle
- Organoheterocyclic compound
- Azacycle
- Organopnictogen compound
- Amine
- Alcohol
- Organic oxide
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Primary amine
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | 215-218 oC | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 200 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | G563 |
|---|