| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:14 UTC |
|---|
| Update date | 2017-01-19 02:36:27 UTC |
|---|
| FoodComEx ID | PC000533 |
|---|
| FoodDB Record | FDB022722 |
|---|
| Chemical Information |
|---|
| Name | Valproic acid |
|---|
| Description | Valproic acid (VPA) is considered to be a drug of first choice and one of the most frequently-prescribed antiepileptic drugs worldwide for the therapy of generalized and focal epilepsies, including special epileptic. It is a broad-spectrum antiepileptic drug and is usually well tolerated. Rarely, serious complications may occur in some patients, including hemorrhagic pancreatitis, coagulopathies, bone marrow suppression, VPA-induced hepatotoxicity and encephalopathy, but there is still a lack of knowledge about the incidence and occurrence of these special side effects. VPA has been approved for stabilization of manic episodes in patients with bipolar disorder. It is also used to treat migraine headaches and schizophrenia. As the use of VPA increases, the number of both accidental and intentional exposures increases. This is paralleled by more reports of VPA-induced toxicity. VPA is relatively contraindicated in pregnancy due to its teratogenicity. It is a known folate antagonist, which can cause neural tube defects. Thus, folic acid supplements may alleviate teratogenic problems. Women who become pregnant whilst taking valproate should be counselled as to its risks. VPA is an inhibitor of the enzyme histone deacetylase 1 (HDAC1). HDAC1 is needed for HIV to remain in infected cells. Patients treated with valproic acid in addition to highly active antiretroviral therapy (HAART) showed a median 75% reduction in latent HIV infection. VPA is believed to affect the function of the neurotransmitter GABA (as a GABA transaminase inhibitor) in the human brain. Valproic Acid dissociates to the valproate ion in the gastrointestinal tract. (PMID: 18201150, 17496767) [HMDB] |
|---|
| CAS Number | 99-66-1 |
|---|
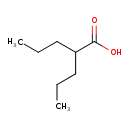
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (n-C3H7)2CHCOOH | hmdb | | (S)-2-propyl-4-pentanoate | hmdb | | (S)-2-propyl-4-pentanoic acid | hmdb | | 2 PP (base) | hmdb | | 2-N-Propyl-N-valerate | Generator | | 2-n-Propyl-n-valeric acid | hmdb | | 2-propyl-Pentanoate | hmdb | | 2-propyl-Pentanoic acid | hmdb | | 2-Propylpentanoate | hmdb | | 2-Propylpentanoic acid | hmdb | | 2-propylpentanoic acid (ACD/Name 4.0) | hmdb | | 2-Propylvalerate | Generator | | 2-Propylvaleric acid | hmdb | | 4-Heptanecarboxylate | Generator | | 4-Heptanecarboxylic acid | hmdb | | Acide valproique | ChEBI | | acide valproique [inn-french] | hmdb | | Acido valproico | ChEBI | | acido valproico [inn-spanish] | hmdb | | Acidum valproicum | ChEBI | | acidum valproicum [inn-latin] | hmdb | | Alti-valproic | hmdb | | APO-divalproex | hmdb | | Apo-valproic | hmdb | | Apo-valproic syrup | hmdb | | Avugane | hmdb | | Baceca | hmdb | | Convulex | hmdb | | Delepsine | hmdb | | Depakene | hmdb | | Depakene Cap 250mg | hmdb | | Depakene Cap 500mg | hmdb | | Depakin | hmdb | | Depakin chrono | hmdb | | Depakine | hmdb | | Depakine chrono | hmdb | | Deproic | hmdb | | Di-N-propylacetate | Generator | | Di-n-propylacetic acid | hmdb | | Di-N-propylessigsaeure | ChEBI | | Di-n-propylessigsaure | hmdb | | Di-n-propylessigsaure [german] | hmdb | | Dipropylacetate | hmdb | | Dipropylacetic acid | hmdb | | Divalproex | hmdb | | Divalproex-125 | hmdb | | Divalproex-250 | hmdb | | Divalproex-500 | hmdb | | DOM-divalproex | hmdb | | Dom-valproate | hmdb | | DOM-valproic | hmdb | | Dom-valproic acid | hmdb | | DOM-valproic acid E.C. | hmdb | | Dom-valproic acid syrup | hmdb | | DPA (van) | hmdb | | Epiject I.V. | hmdb | | Epilex | hmdb | | Epilim | hmdb | | Epival | hmdb | | Epival Ect 125mg | hmdb | | Epival Ect 250mg | hmdb | | Epival Ect 500mg | hmdb | | Epival er | hmdb | | Ergenyl | hmdb | | Gen-divalproex | hmdb | | Gen-Valproic - Cap 250mg | hmdb | | Kyselina 2-propylvalerova | hmdb | | Kyselina 2-propylvalerova [Czech] | hmdb | | Med valproic | hmdb | | Mylproin | hmdb | | N-dipropylacetic acid | hmdb | | N-DPA | hmdb | | nchembio815-comp21 | hmdb | | Novo-divalproex | hmdb | | Novo-valproic | hmdb | | Novo-Valproic - ECC | hmdb | | Novo-valproic soft gel cap | hmdb | | Nu-Divalproex 125mg | hmdb | | Nu-Divalproex 250mg | hmdb | | Nu-Divalproex 500mg | hmdb | | Nu-valproic | hmdb | | PEAC | hmdb | | Penta-valproic | hmdb | | PHL-valproate | hmdb | | PHL-valproic acid | hmdb | | PHL-valproic acid E.C. | hmdb | | PMS-Divalproex | hmdb | | PMS-Divalproex (125mg) | hmdb | | PMS-Divalproex (500mg) | hmdb | | PMS-valproate | hmdb | | PMS-valproic acid | hmdb | | PMS-valproic acid E.C. | hmdb | | Propylvaleric acid | hmdb | | Ratio-Valproic - ECC | hmdb | | Ratio-Valproic 50mg/mL Syrup | hmdb | | Ratio-Valproic-Cap 250mg | hmdb | | S-2-n-Propyl-4-pentenoate | hmdb | | S-2-n-Propyl-4-pentenoic acid | hmdb | | S(-)-4-En-valproate | hmdb | | S(-)-4-En-valproic acid | hmdb | | Sandoz valproic | hmdb | | Savicol | hmdb | | Sodium hydrogen divalproate | hmdb | | Sodium hydrogen divalproic acid | hmdb | | Sprinkle | hmdb | | Valcote | hmdb | | Valparin | hmdb | | Valproate semisodique [french] | hmdb | | Valproate semisodium | hmdb | | Valproato semisodico [spanish] | hmdb | | Valproatum seminatricum [latin] | hmdb | | Valproic acid [usan:ban:inn] | hmdb | | Valproic acid USP | hmdb | | Valproic acid USP24 | hmdb | | Valproic-250 | hmdb | | Valproinsaeure | ChEBI | | VPA | hmdb |
|
|---|
| Chemical Formula | Not Available |
|---|
| IUPAC name | Not Available |
|---|
| InChI Identifier | InChI=1S/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10) |
|---|
| InChI Key | NIJJYAXOARWZEE-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CCCC(CCC)C(O)=O |
|---|
| Average Molecular Weight | 144.2114 |
|---|
| Monoisotopic Molecular Weight | 144.115029756 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as methyl-branched fatty acids. These are fatty acids with an acyl chain that has a methyl branch. Usually, they are saturated and contain only one or more methyl group. However, branches other than methyl may be present. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Methyl-branched fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Methyl-branched fatty acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | B396 |
|---|
| AKSci | J91459 |
|---|
| Toronto Research Chemicals | V094750 |
|---|