| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:12 UTC |
|---|
| Update date | 2017-01-19 02:36:27 UTC |
|---|
| FoodComEx ID | PC000530 |
|---|
| FoodDB Record | FDB012307 |
|---|
| Chemical Information |
|---|
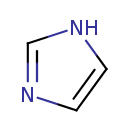
| Name | Imidazole |
|---|
| Description | Isolated from the seeds of Lens culinaris (lentil)and is also present in the seeds of other legumes: Macrotyloma uniflorum (horse gram), Psophocarpus tetragonolobus (winged bean), Vigna radiata (mung bean)
Imidazole is a 5-membered planar ring, which is soluble in water and other polar solvents. It exists in two equivalent tautomeric forms because the hydrogen atom can be located on either of the two nitrogen atoms. Imidazole is a highly polar compound, as evidenced by a calculated dipole of 3.61D, and is entirely soluble in water. The compound is classified as aromatic due to the presence of a sextet of ?-electrons, consisting of a pair of electrons from the protonated nitrogen atom and one from each of the remaining four atoms of the ring.; Imidazole is a heterocyclic aromatic organic compound. It is further classified as an alkaloid. Imidazole refers to the parent compound C3H4N2, while imidazoles are a class of heterocycles with similar ring structure but varying substituents. This ring system is present in important biological building blocks such as histidine and histamine. Imidazole can act as a base and as a weak acid. Imidazole exists in two tautomeric forms with the hydrogen atom moving between the two nitrogens. Many drugs contain an imidazole ring, such as antifungal drugs and nitroimidazole. Imidazole is a 5 membered planar ring which is soluble in water and polar solvents. Imidazole is a base and an excellent nucleophile. It reacts at the NH nitrogen, attacking alkylating and acylating compounds. It is not particularly susceptible to electrophilic attacks at the carbon atoms, and most of these reactions are substitutions that keep the aromaticity intact. One can see from the resonance structure that the carbon-2 is the carbon most likely to have a nucleophile attack it, but in general nucleophilic substitutions are difficult with imidazole. Imidazole is incorporated into many important biological molecules. The most obvious is the amino acid histidine, which has an imidazole side chain. histidine is present in many proteins and enzymes and plays a vital part in the structure and binding functions of hemoglobin.; Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is classified as an alkaloid. Imidazole refers to the parent compound whereas imidazoles are a class of heterocycles with similar ring structure but varying substituents. This ring system is present in important biological building blocks such as histidine, and the related hormone histamine. Imidazole can serve as a base and as a weak acid. Many drugs contain an imidazole ring, such as antifungal drugs and nitroimidazole.; Imidazole is incorporated into many important biological molecules. The most pervasive is the amino acid histidine, which has an imidazole side chain. Histidine is present in many proteins and enzymes and plays a vital part in the structure and binding functions of hemoglobin. Histidine can be decarboxylated to histamine, which is also a common biological compound. It is a component of the toxin that causes urticaria, which is another name for allergic hives. The relationship between histidine and histamine are shown below:; This is a general method which is able to give good yields for substituted imidazoles. It is essentially an adaptation of the Debus method called the Debus-Radziszewski imidazole synthesis. The starting materials are substituted glyoxal, aldehyde, amine, and ammonia or an ammonium salt. Imidazole is found in pulses. |
|---|
| CAS Number | 288-32-4 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| {Pyrro[b]monazole} | HMDB | | 1,3-Diaza-2,4-cyclopentadiene | biospider | | 1,3-Diaza-2,4-cyclopentadiene- | biospider | | 1,3-Diazole | db_source | | 1467-16-9 (mono-hydrochloride) | biospider | | 1H-Imidazole | biospider | | 1H-Imidazole (9CI) | biospider | | Formamidine, n,n'-vinylene- | biospider | | Glioksal | biospider | | Glyoxalin | biospider | | Glyoxaline | db_source | | HIM | biospider | | IMD | biospider | | Imidazol | biospider | | Imidazole (8CI) | biospider | | Imidazole-ring | biospider | | Iminazole | db_source | | Imutex | biospider | | Methanimidamide, N,N'-1, 2-ethenediyl- | biospider | | Methanimidamide, N,N'-1,2-ethenediyl- | biospider | | Miazole | biospider | | N,N'-1,2-ethenediylmethanimidamide | biospider | | N,n'-vinyleneformamidine | biospider | | Pyrro(b)monazole | biospider | | Pyrro[b]monazole | biospider |
|
|---|
| Chemical Formula | C3H4N2 |
|---|
| IUPAC name | 1H-imidazole |
|---|
| InChI Identifier | InChI=1S/C3H4N2/c1-2-5-3-4-1/h1-3H,(H,4,5) |
|---|
| InChI Key | RAXXELZNTBOGNW-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | N1C=CN=C1 |
|---|
| Average Molecular Weight | 68.0773 |
|---|
| Monoisotopic Molecular Weight | 68.037448138 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as imidazoles. Imidazoles are compounds containing an imidazole ring, which is an aromatic five-member ring with two nitrogen atoms at positions 1 and 3, and three carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Imidazoles |
|---|
| Direct Parent | Imidazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Heteroaromatic compound
- Imidazole
- Azacycle
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -0.08 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 88-90° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | D070 |
|---|
| AKSci | J70047 |
|---|
| Glentham | GB9580 |
|---|
| MetaSci | HMDB0001525 |
|---|
| Sigma-Aldrich | HMDB0001525 |
|---|
| Toronto Research Chemicals | I350200 |
|---|