| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:10 UTC |
|---|
| Update date | 2017-01-19 02:36:27 UTC |
|---|
| FoodComEx ID | PC000528 |
|---|
| FoodDB Record | FDB002237 |
|---|
| Chemical Information |
|---|
| Name | Trigonelline |
|---|
| Description | N-Methylnicotinate or Trigonelline, also known as caffearin or gynesine, belongs to the class of organic compounds known as alkaloids and derivatives. These are naturally occurring chemical compounds that contain mostly basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and more rarely other elements such as chlorine, bromine, and phosphorus. Trigonelline is a moderately acidic compound. Trigonelline exists in all living organisms, from bacteria to humans. It is a product of the metabolism of niacin (vitamin B3) and it is excreted in the urine. Trigonelline in urine is a biomarker for the consumption of coffee, legumes and soy products. Trigonelline is found in highest concentrations in arabica coffee, fenugreeks, and common pea and in lower concentrations in yellow bell peppers, orange bell peppers, and muskmelons. Trigonelline has also been detected in rices, triticales, alfalfa, cereals and cereal products, ryes fenugreek seeds, garden peas, oats and potatoes. Trigonelline may reduce dental caries by preventing the bacteria Streptococcus mutans from adhering to teeth. It also has shown anti-carcinogenic and anti-hyperglycemic effects in several studies (PMID: 22680628; PMID: 31896496; PMID: 31363374). |
|---|
| CAS Number | 535-83-1 |
|---|
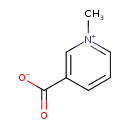
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1-Methyl-3-pyridiniumcarboxylate | biospider | | 1-Methyl-3-pyridiniumcarboxylic acid | Generator | | 1-methylnicotinate | biospider | | 1-Methylnicotinic acid | Generator | | 1-Methylpyridinio-3-carboxylate | biospider | | 1-Methylpyridinio-3-carboxylic acid | Generator | | 1-methylpyridinium-3-carboxylate | biospider | | 3-carboxy-1-methyl-Pyridinium hydroxide inner salt | biospider | | 3-Carboxy-1-methylpyridinium hydroxide inner salt | db_source | | 3-Carboxy-1-methylpyridinium hydroxide, inner salt | biospider | | 535-83-1 (INNER SALT) | biospider | | Betain nicotinate | biospider | | Betain nicotinic acid | Generator | | Betaine nicotinate | biospider | | Betaine nicotinic acid | Generator | | Caffearin | biospider | | Caffearine | biospider | | Coffearin | db_source | | Coffearine | biospider | | Gynesine | biospider | | Gynesis | db_source | | N-methyl-nicotinate | biospider | | N-Methyl-nicotinic acid | Generator | | N-methylnicotinate | biospider | | N-methylnicotinic acid | biospider | | N-Methylnicotinic betaine | db_source | | N'-methylnicotinate | biospider | | N'-methylnicotinic acid | biospider | | Nicotinate N-methylbetaine | Generator | | Nicotinic acid n-methylbetaine | biospider | | Pyridinium, 3-carboxy-1-methyl-, hydroxide, inner salt | biospider | | Pyridinium, 3-carboxy-1-methyl-, inner salt | biospider | | Trigenelline | biospider | | Trigenolline | biospider | | Trigonellin | biospider | | Trigonelline | db_source |
|
|---|
| Chemical Formula | C7H7NO2 |
|---|
| IUPAC name | 1-methylpyridin-1-ium-3-carboxylate |
|---|
| InChI Identifier | InChI=1S/C7H7NO2/c1-8-4-2-3-6(5-8)7(9)10/h2-5H,1H3 |
|---|
| InChI Key | WWNNZCOKKKDOPX-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | C[N+]1=CC=CC(=C1)C([O-])=O |
|---|
| Average Molecular Weight | 137.136 |
|---|
| Monoisotopic Molecular Weight | 137.047678473 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alkaloids and derivatives. These are naturally occurring chemical compounds that contain mostly basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Also some synthetic compounds of similar structure are attributed to alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and more rarely other elements such as chlorine, bromine, and phosphorus. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Not Available |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Alkaloids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyridine carboxylic acid
- Alkaloid or derivatives
- Pyridine carboxylic acid or derivatives
- N-methylpyridinium
- Pyridinium
- Pyridine
- Heteroaromatic compound
- Vinylogous amide
- Carboxylic acid salt
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Organoheterocyclic compound
- Organic nitrogen compound
- Organic salt
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 218 dec. (anhyd.) | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | V1545 |
|---|
| Toronto Research Chemicals | T794000 |
|---|