| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:08 UTC |
|---|
| Update date | 2017-01-19 02:36:27 UTC |
|---|
| FoodComEx ID | PC000525 |
|---|
| FoodDB Record | FDB000565 |
|---|
| Chemical Information |
|---|
| Name | alpha-Tocopherol |
|---|
| Description | Constituent of many vegetable oils such as soya and sunflower oils. Dietary supplement and nutrient. Nutriceutical with anticancer and antioxidant props. Added to fats and oils to prevent rancidity. The naturally-occurring tocopherol is a single stereoisomer; synthetic forms are a mixture of all eight possible isomers
Alpha-tocopherol is traditionally recognized as the most active form of vitamin E in humans, and is a powerful biological antioxidant. alpha-Tocopherol is found in many foods, some of which are custard apple, nopal, bitter gourd, and kumquat. |
|---|
| CAS Number | 59-02-9 |
|---|
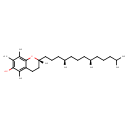
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (+)-a-tocopherol | biospider | | (+)-alpha-tocopherol | biospider | | (+)-α-tocopherol | Generator | | (2R,4'R,8'R)-a-Tocopherol | biospider | | (2R,4'R,8'R)-alpha-Tocopherol | biospider | | (2R,4'R,8'r)-α-tocopherol | Generator | | (2R)-3,4-dihydro-2,5,7,8-Tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-2H-1-benzopyran-6-ol | HMDB | | (all-r)-a-tocopherol | biospider | | (r,r,r)-a-tocopherol | biospider | | (r,r,r)-alpha-tocopherol | biospider | | (R,R,R)-α-tocopherol | Generator | | 3,4-Dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2H-1-benzopyran-6-ol, 9CI | db_source | | 5,7,8-Trimethyltocol | db_source | | a-D-Tocopherol | HMDB | | a-tocopherol | biospider | | Alpha-delta-tocopherol | biospider | | Alpha-tocopherol | biospider | | alpha-Vitamin E | manual | | Antisterility vitamin | db_source | | Covitol | biospider | | D-a-Tocopherol | Generator | | D-alpha-tocopherol | biospider | | D-α-tocopherol | Generator | | Delta-alpha-tocopherol | biospider | | Denamone | biospider | | E307 | db_source | | Emipherol | biospider | | Ephanyl | biospider | | Ephynal | db_source | | Eprolin | biospider | | Natopherol | biospider | | Phytogermin | biospider | | Phytogermine | HMDB | | Profecundin | db_source | | RRR-alpha-tocopherol | biospider | | RRR-alpha-tocopheryl | HMDB | | Syntopherol | db_source | | Tocopherol | biospider | | Vitamin e | ChEBI | | Vitamin E? | db_source | | Vitamin ea | HMDB | | α-tocopherol | Generator |
|
|---|
| Chemical Formula | C29H50O2 |
|---|
| IUPAC name | (2R)-2,5,7,8-tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydro-2H-1-benzopyran-6-ol |
|---|
| InChI Identifier | InChI=1S/C29H50O2/c1-20(2)12-9-13-21(3)14-10-15-22(4)16-11-18-29(8)19-17-26-25(7)27(30)23(5)24(6)28(26)31-29/h20-22,30H,9-19H2,1-8H3/t21-,22-,29-/m1/s1 |
|---|
| InChI Key | GVJHHUAWPYXKBD-IEOSBIPESA-N |
|---|
| Isomeric SMILES | CC(C)CCC[C@@H](C)CCC[C@@H](C)CCC[C@]1(C)CCC2=C(O1)C(C)=C(C)C(O)=C2C |
|---|
| Average Molecular Weight | 430.7061 |
|---|
| Monoisotopic Molecular Weight | 430.381080844 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as tocopherols. These are vitamin E derivatives containing a saturated trimethyltridecyl chain attached to the carbon C6 atom of a benzopyran ring system. The differ from tocotrienols that contain an unsaturated trimethyltrideca-3,7,11-trien-1-yl chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Quinone and hydroquinone lipids |
|---|
| Direct Parent | Tocopherols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tocopherol
- Diterpenoid
- 1-benzopyran
- Benzopyran
- Chromane
- Alkyl aryl ether
- Benzenoid
- Oxacycle
- Organoheterocyclic compound
- Ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 2.5-3.5° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | liquid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Toronto Research Chemicals | T526125 |
|---|