| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:08 UTC |
|---|
| Update date | 2017-01-19 02:36:26 UTC |
|---|
| FoodComEx ID | PC000523 |
|---|
| FoodDB Record | FDB022720 |
|---|
| Chemical Information |
|---|
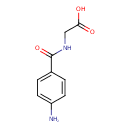
| Name | 4-Aminohippuric acid |
|---|
| Description | 4-Aminohippuric acid is an acyl glycine. Acyl glycines are normally minor metabolites of fatty acids. However, the excretion of certain acyl glycines is increased in several inborn errors of metabolism. In certain cases the measurement of these metabolites in body fluids can be used to diagnose disorders associated with mitochondrial fatty acid beta-oxidation. Acyl glycines are produced through the action of glycine N-acyltransferase (EC 2.3.1.13) which is an enzyme that catalyzes the chemical reaction:
acyl-CoA + glycine < -- > CoA + N-acylglycine
Renal proximal tubules secrete various organic anions, including drugs and p-aminohippurate (PAH). Uptake of PAH from blood into tubule cells occurs by exchange with intracellular alpha-ketoglutarate and is mediated by the organic anion transporter 1. PAH exit into tubule lumen is species specific and may involve ATP-independent and -dependent transporters. (PMID 11443229)
Enhanced secretion of p-aminohippuric acid occurs in Fanconi's syndrome (FS). FS is associated with numerous varieties of inherited and acquired conditions; FS is characterized by a generalized transport defect in the proximal tubules, leading to renal losses of glucose, phosphate, calcium, uric acid, amino acids, bicarbonates, and other organic compounds. (PMID 12552490) [HMDB] |
|---|
| CAS Number | 61-78-9 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 4-Aminohippate | Generator | | 4-Aminohippic acid | Generator | | 4-Aminohippurate | hmdb | | Aminohippate | Generator | | Aminohippic acid | Generator | | Aminohippurate | hmdb | | Aminohippuric acid | hmdb | | Chlorphentermine | hmdb | | N-(4-Aminobenzoyl)glycine | hmdb | | N-(p-Aminobenzoyl)aminoacetate | hmdb | | N-(p-Aminobenzoyl)aminoacetic acid | hmdb | | N-(p-Aminobenzoyl)glycine | hmdb | | N-(para-aminobenzoyl)glycine | hmdb | | Nefrotest | hmdb | | P-Aminohippate | Generator | | P-Aminohippic acid | Generator | | p-Aminohippurate | hmdb | | p-Aminohippuric acid | hmdb | | PAH | hmdb | | Paha | hmdb | | Para-aminohippate | Generator | | Para-aminohippic acid | Generator | | para-Aminohippurate | hmdb | | para-Aminohippuric acid | hmdb | | Paraaminohippate | Generator | | Paraaminohippic acid | Generator | | paraaminohippuric acid | hmdb |
|
|---|
| Chemical Formula | C9H10N2O3 |
|---|
| IUPAC name | 2-[(4-aminophenyl)formamido]acetic acid |
|---|
| InChI Identifier | InChI=1S/C9H10N2O3/c10-7-3-1-6(2-4-7)9(14)11-5-8(12)13/h1-4H,5,10H2,(H,11,14)(H,12,13) |
|---|
| InChI Key | HSMNQINEKMPTIC-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NC1=CC=C(C=C1)C(=O)NCC(O)=O |
|---|
| Average Molecular Weight | 194.1873 |
|---|
| Monoisotopic Molecular Weight | 194.069142196 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as hippuric acids. Hippuric acids are compounds containing hippuric acid, which consists of a of a benzoyl group linked to the N-terminal of a glycine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Hippuric acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hippuric acid
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid or derivatives
- Aminobenzamide
- Aminobenzoic acid or derivatives
- Benzoyl
- Aniline or substituted anilines
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Carboxamide group
- Amino acid
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Primary amine
- Amine
- Organonitrogen compound
- Carbonyl group
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic oxide
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | X6859 |
|---|
| Cayman Chemical | 23726 |
|---|
| Toronto Research Chemicals | A627970 |
|---|