| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:07 UTC |
|---|
| Update date | 2017-01-19 02:36:26 UTC |
|---|
| FoodComEx ID | PC000517 |
|---|
| FoodDB Record | FDB021903 |
|---|
| Chemical Information |
|---|
| Name | Anserine |
|---|
| Description | This dipeptide is normally absent from human tissues and body fluids, and its appearance there is an artifact of diet (Proc Soc Pediatr Res 134, 1967.) and serum carnosinase deficiency. (OMIM 212200) Anserine is present in the skeletal muscle of birds and certain species of mammals, notably the rabbit, rat, and whale, contains anserine. (Proc Soc Pediatr Res 134, 1967) The methyl group of anserine is added to carnosine by the enzyme S-adenosylmethionine: carnosine N-methyltransferase. (J Biol Chem 237:1207, 1962.) [HMDB]. Anserine is a biomarker for the consumption of meat, especially chicken. |
|---|
| CAS Number | 584-85-0 |
|---|
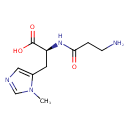
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| Anserine | hmdb | | b-Alanyl-3-methyl-L-histidine | Generator | | b-Alanyl-N(pai)-methyl-L-histidine | Generator | | beta-Alanyl-3-methyl-L-histidine | ChEBI | | beta-alanyl-N(pai)-methyl-L-histidine | hmdb | | L-Anserine | hmdb | | L-N-b-alanyl-3-methyl-Histidine | hmdb | | L-N-beta-alanyl-3-methyl-Histidine | hmdb | | N-b-alanyl-3-methyl-L-Histidine | hmdb | | N-beta-Alanyl-3-methyl-L-histidine | hmdb | | β-alanyl-3-methyl-L-histidine | Generator | | β-alanyl-N(pai)-methyl-L-histidine | Generator |
|
|---|
| Chemical Formula | C10H16N4O3 |
|---|
| IUPAC name | (2S)-2-(3-aminopropanamido)-3-(1-methyl-1H-imidazol-5-yl)propanoic acid |
|---|
| InChI Identifier | InChI=1S/C10H16N4O3/c1-14-6-12-5-7(14)4-8(10(16)17)13-9(15)2-3-11/h5-6,8H,2-4,11H2,1H3,(H,13,15)(H,16,17)/t8-/m0/s1 |
|---|
| InChI Key | MYYIAHXIVFADCU-QMMMGPOBSA-N |
|---|
| Isomeric SMILES | CN1C=NC=C1C[C@H](NC(=O)CCN)C(O)=O |
|---|
| Average Molecular Weight | 240.259 |
|---|
| Monoisotopic Molecular Weight | 240.122240398 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as hybrid peptides. Hybrid peptides are compounds containing at least two different types of amino acids (alpha, beta, gamma, delta) linked to each other through a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Peptidomimetics |
|---|
| Sub Class | Hybrid peptides |
|---|
| Direct Parent | Hybrid peptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hybrid peptide
- Histidine or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid or derivatives
- Imidazolyl carboxylic acid derivative
- N-substituted imidazole
- Azole
- Imidazole
- Heteroaromatic compound
- Amino acid or derivatives
- Amino acid
- Carboximidic acid
- Carboximidic acid derivative
- Carboxylic acid derivative
- Carboxylic acid
- Azacycle
- Monocarboxylic acid or derivatives
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Primary aliphatic amine
- Carbonyl group
- Organic nitrogen compound
- Amine
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 5 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | X6637 |
|---|