| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:30:58 UTC |
|---|
| Update date | 2017-01-19 02:36:26 UTC |
|---|
| FoodComEx ID | PC000503 |
|---|
| FoodDB Record | FDB022631 |
|---|
| Chemical Information |
|---|
| Name | Lipoic acid |
|---|
| Description | A vitamin-like antioxidant that acts as a free-radical scavenger. Alpha-lipoic acid is also known as thioctic acid. It is a naturally occurring compound that is synthesized by both plants and animals. Lipoic acid contains two thiol groups which may be either oxidized or reduced. The reduced form is known as dihydrolipoic acid (DHLA). Lipoic acid (Delta E= -0.288) is therefore capable of thiol-disulfide exchange, giving it antioxidant activity. Lipoate is a critical cofactor for aerobic metabolism, participating in the transfer of acyl or methylamine groups via the 2-Oxoacid dehydrogenase (2-OADH) or alpha-ketoglutarate dehydrogenase complex. This enzyme catalyzes the conversion of alpha-ketoglutarate to succinyl CoA. This activity results in the catabolism of the branched chain amino acids (leucine, isoleucine and valine). Lipoic acid also participates in the glycine cleavage system(GCV). The glycine cleavage system is a multi-enzyme complex that catalyzes the oxidation of glycine to form 5,10 methylene tetrahydrofolate, an important cofactor in nucleic acid synthesis. Since Lipoic acid is an essential cofactor for many enzyme complexes, it is essential for aerobic life as we know it. This system is used by many organisms and plays a crucial role in the photosynthetic carbon cycle. Lipoic acid was first postulated to be an effective antioxidant when it was found it prevented vitamin C and vitamin E deficiency. It is able to scavenge reactive oxygen species and reduce other metabolites, such as glutathione or vitamins, maintaining a healthy cellular redox state. Lipoic acid has been shown in cell culture experiments to increase cellular uptake of glucose by recruiting the glucose transporter GLUT4 to the cell membrane, suggesting its use in diabetes. Studies of rat aging have suggested that the use of L-carnitine and lipoic acid results in improved memory performance and delayed structural mitochondrial decay. As a result, it may be helpful for people with Alzheimer's disease or Parkinson's disease. -- Wikipedia [HMDB] |
|---|
| CAS Number | 1200-22-2 |
|---|
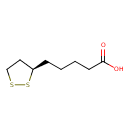
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (+-)-1,2-Dithiolane-3-pentanoate | hmdb | | (+-)-1,2-Dithiolane-3-pentanoic acid | hmdb | | (+-)-1,2-Dithiolane-3-valerate | hmdb | | (+-)-1,2-Dithiolane-3-valeric acid | hmdb | | (+)-alpha-Lipoate | hmdb | | (+)-alpha-Lipoic acid | hmdb | | (R)-1,2-Dithiolane-3-pentanoate | hmdb | | (R)-1,2-Dithiolane-3-pentanoic acid | hmdb | | (RS)-alpha-Lipoate | hmdb | | (RS)-alpha-Lipoic acid | hmdb | | (RS)-Lipoate | hmdb | | (RS)-Lipoic acid | hmdb | | (S)-(−)-lipoic acid | HMDB | | (S)-1,2-Dithiolane-3-pentanoic acid | HMDB | | (S)-alpha-Lipoic acid | HMDB | | 1,2-Dithiolane-3-pentanoate | hmdb | | 1,2-Dithiolane-3-pentanoic acid | hmdb | | 1,2-Dithiolane-3-valerate | hmdb | | 1,2-Dithiolane-3-valeric acid | hmdb | | 1,2-Dithiolane-3R-pentanoate | hmdb | | 1,2-Dithiolane-3R-pentanoic acid | hmdb | | 5-(1,2-Dithiolan-3-yl)pentanoate | hmdb | | 5-(1,2-Dithiolan-3-yl)pentanoic acid | hmdb | | 5-(1,2-Dithiolan-3-yl)valerate | hmdb | | 5-(1,2-Dithiolan-3-yl)valeric acid | hmdb | | 5-(Dithiolan-3-yl)valerate | hmdb | | 5-(Dithiolan-3-yl)valeric acid | hmdb | | 5-[3-(1,2-dithiolanyl)]pentanoate | hmdb | | 5-[3-(1,2-dithiolanyl)]pentanoic acid | hmdb | | 6-Thioctate | hmdb | | 6-Thioctic acid | hmdb | | 6-Thiotate | hmdb | | 6-Thiotic acid | hmdb | | 6,8-Dithiooctanoate | hmdb | | 6,8-Dithiooctanoic acid | hmdb | | 6,8-Thioctate | hmdb | | 6,8-Thioctic acid | hmdb | | 6,8-Thiotate | hmdb | | 6,8-Thiotic acid | hmdb | | acetate replacing factor | hmdb | | Acetate-replacing factor | hmdb | | alpha Lipoate | hmdb | | alpha Lipoic acid | hmdb | | alpha-Liponate | hmdb | | alpha-Liponic acid | hmdb | | alpha-Liponsaeure | hmdb | | Biletan | hmdb | | delta-[3-(1,2-dithiacyclopentyl)]pentanoate | hmdb | | delta-[3-(1,2-dithiacyclopentyl)]pentanoic acid | hmdb | | DL-1,2-Dithiolane 3-valerate | hmdb | | DL-1,2-Dithiolane 3-valeric acid | hmdb | | DL-6-Thioctate | hmdb | | DL-6-Thioctic acid | hmdb | | DL-6,8-Dithiooctanoate | hmdb | | DL-6,8-Dithiooctanoic acid | hmdb | | DL-6,8-Thioctate | hmdb | | DL-6,8-Thioctic acid | hmdb | | DL-alpha-Lipoate | hmdb | | DL-alpha-Lipoic acid | hmdb | | dl-Lipoate | hmdb | | dl-Lipoic acid | hmdb | | dl-Thioctate | hmdb | | dl-Thioctic acid | hmdb | | DL-Thioctic acid > 98% | hmdb | | Heparlipon | hmdb | | L-1,2-Dithiolane 3-valeric acid | HMDB | | L-6-Thioctic acid | HMDB | | L-6,8-Thioctic acid | HMDB | | Lip | hmdb | | Lipoate | hmdb | | Lipoic acid | hmdb | | liponate | hmdb | | liponic acid | hmdb | | Liposan | hmdb | | Lipothion | hmdb | | Protogen A | hmdb | | Pyruvate oxidation factor | hmdb | | R-Lipoate | hmdb | | R-Lipoic acid | hmdb | | Rac-lipoate | hmdb | | Rac-lipoic acid | hmdb | | S-LA | HMDB | | SLA | HMDB | | Thioctacid | hmdb | | Thioctan | hmdb | | Thioctate | hmdb | | Thioctic acid | hmdb | | Thioctic acid d-form | hmdb | | Thioctic acid dl-form | hmdb | | Thioctic acid L-form | HMDB | | Thioctidase | hmdb | | Thioctsan | hmdb | | Thioktsaeure | hmdb | | Thiooctanoate | hmdb | | Thiooctanoic acid | hmdb | | Tioctacid | hmdb | | Tioctan | hmdb | | Tioctidasi | hmdb | | Tioctidasi acetate replacing factor | hmdb |
|
|---|
| Chemical Formula | C8H14O2S2 |
|---|
| IUPAC name | 5-[(3R)-1,2-dithiolan-3-yl]pentanoic acid |
|---|
| InChI Identifier | InChI=1S/C8H14O2S2/c9-8(10)4-2-1-3-7-5-6-11-12-7/h7H,1-6H2,(H,9,10)/t7-/m1/s1 |
|---|
| InChI Key | AGBQKNBQESQNJD-SSDOTTSWSA-N |
|---|
| Isomeric SMILES | OC(=O)CCCC[C@@H]1CCSS1 |
|---|
| Average Molecular Weight | 206.326 |
|---|
| Monoisotopic Molecular Weight | 206.043521072 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as lipoic acids and derivatives. Lipoic acids and derivatives are compounds containing a lipoic acid moiety (or a derivative thereof), which consists of a pentanoic acid (or derivative) attached to the C3 carbon atom of a 1,2-dithiolane ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Dithiolanes |
|---|
| Sub Class | Lipoic acids and derivatives |
|---|
| Direct Parent | Lipoic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Lipoic_acid_derivative
- Medium-chain fatty acid
- Heterocyclic fatty acid
- Thia fatty acid
- Fatty acyl
- Fatty acid
- 1,2-dithiolane
- Organic disulfide
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | H452 |
|---|