| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:30:58 UTC |
|---|
| Update date | 2017-01-19 02:36:26 UTC |
|---|
| FoodComEx ID | PC000499 |
|---|
| FoodDB Record | FDB000938 |
|---|
| Chemical Information |
|---|
| Name | 3-(1H-Indol-3-yl)-2-propenoic acid |
|---|
| Description | Major auxin from roots of Lens culinaris (lentil)
A natural auxin from lentil roots. Inhibits the growth of mycelia of Neurospora crassa and causes the cells to accumulate indoleglycerol phosphate. 3-(1H-Indol-3-yl)-2-propenoic acid is found in lentils and pulses. |
|---|
| CAS Number | 1204-06-4 |
|---|
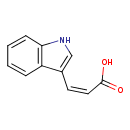
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1H-Indole-3-propenoic acid | db_source | | 2-Propenoic acid, 3-(1-H-indol-3-yl) | biospider | | 2-Propenoic acid, 3-(1H-indol-3-yl)- | biospider | | 3-(3-Indolyl)acrylic acid | db_source | | 3-(Indol-3-yl)acrylic acid | biospider | | 3-indoleacrylate | biospider | | 3-indoleacrylic acid | biospider | | 3-Indolylacrylic acid | biospider | | Indole-3-acrylic acid | biospider | | Indole-3β-acrylic acid | biospider | | Indole-3beta-acrylic acid | biospider | | Indoleacrylate | biospider | | Indoleacrylic acid | biospider |
|
|---|
| Chemical Formula | C11H9NO2 |
|---|
| IUPAC name | (2Z)-3-(1H-indol-3-yl)prop-2-enoic acid |
|---|
| InChI Identifier | InChI=1S/C11H9NO2/c13-11(14)6-5-8-7-12-10-4-2-1-3-9(8)10/h1-7,12H,(H,13,14)/b6-5- |
|---|
| InChI Key | PLVPPLCLBIEYEA-WAYWQWQTSA-N |
|---|
| Isomeric SMILES | OC(=O)\C=C/C1=CNC2=C1C=CC=C2 |
|---|
| Average Molecular Weight | 187.1947 |
|---|
| Monoisotopic Molecular Weight | 187.063328537 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as indoles. Indoles are compounds containing an indole moiety, which consists of pyrrole ring fused to benzene to form 2,3-benzopyrrole. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Indoles |
|---|
| Direct Parent | Indoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Indole
- Substituted pyrrole
- Benzenoid
- Heteroaromatic compound
- Pyrrole
- Azacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | 180 - 186 oC | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 300 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |