| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:30:49 UTC |
|---|
| Update date | 2017-01-19 02:36:25 UTC |
|---|
| FoodComEx ID | PC000468 |
|---|
| FoodDB Record | FDB008305 |
|---|
| Chemical Information |
|---|
| Name | (E)-Aconitic acid |
|---|
| Description | Isolated from Asarum europaeum, from cane-sugar molasses, roasted chicory root, roasted malt barley, passion fruit, sorghum root and sugar beet. Flavouring agent used in fruit flavours and alcoholic beverages. Aconitic acid is an organic acid. The two isomers are cis-aconitic acid and trans-aconitic acid. The conjugate base of cis-aconitic acid, cis-aconitate is an intermediate in the isomerisation of citrate to isocitrate in the citric acid cycle. It is acted upon by aconitase. Trans-aconitate in the urine is a biomarker for the consumption of soy products. (E)-Aconitic acid is found in many foods, some of which are cereals and cereal products, rice, garden tomato (variety), and root vegetables. |
|---|
| CAS Number | 4023-65-8 |
|---|
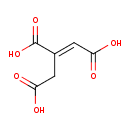
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (1e)-1-Propene-1,2,3-tricarboxylate | Generator | | (1E)-1-Propene-1,2,3-tricarboxylic acid | biospider | | (1e)-Prop-1-ene-1,2,3-tricarboxylic | HMDB | | (1E)-Prop-1-ene-1,2,3-tricarboxylic acid | biospider | | (1e)1-Propene-1,2,3-tricarboxylate | HMDB | | (1e)1-Propene-1,2,3-tricarboxylic acid | HMDB | | (e)-1-Propene-1,2,3-tricarboxylate | Generator | | (E)-1-Propene-1,2,3-tricarboxylic acid | biospider | | (E)-Aconitic acid | biospider | | 1-Propene-1-trans-2,3-tricarboxylic acid | biospider | | 1-Propene-1,2,3-tricarboxylic acid, (1E)- | biospider | | 1-Propene-1,2,3-tricarboxylic acid, (E)- (8CI)(9CI) | biospider | | 1-trans-Propene-1,2,3-tricarboxylic acid | biospider | | Acid | HMDB | | Aconitic acid, trans | biospider | | TRA | HMDB | | trans-1-Propene-1,2,3-tricarboxylic acid | biospider | | trans-Aconitate | biospider | | trans-Aconitic acid | biospider | | trans-Propene-1,2,3-tricarboxylic acid | biospider |
|
|---|
| Chemical Formula | C6H6O6 |
|---|
| IUPAC name | (1E)-prop-1-ene-1,2,3-tricarboxylic acid |
|---|
| InChI Identifier | InChI=1S/C6H6O6/c7-4(8)1-3(6(11)12)2-5(9)10/h1H,2H2,(H,7,8)(H,9,10)(H,11,12)/b3-1+ |
|---|
| InChI Key | GTZCVFVGUGFEME-HNQUOIGGSA-N |
|---|
| Isomeric SMILES | OC(=O)C\C(=C/C(O)=O)C(O)=O |
|---|
| Average Molecular Weight | 174.1082 |
|---|
| Monoisotopic Molecular Weight | 174.016437924 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as tricarboxylic acids and derivatives. These are carboxylic acids containing exactly three carboxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Tricarboxylic acids and derivatives |
|---|
| Direct Parent | Tricarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tricarboxylic acid or derivatives
- Carboxylic acid
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 194-195° dec. | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | D582 |
|---|
| Fluka | HMDB0000958 |
|---|
| MetaSci | HMDB0000958 |
|---|
| Toronto Research Chemicals | A189885 |
|---|