| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:30:49 UTC |
|---|
| Update date | 2017-01-19 02:36:25 UTC |
|---|
| FoodComEx ID | PC000465 |
|---|
| FoodDB Record | FDB021962 |
|---|
| Chemical Information |
|---|
| Name | 2-Hydroxyestradiol |
|---|
| Description | 2-Hydroxyestradiol is generated from estradiol by a Cytochrome P450. 2-Hydroxyestradiol binds, with a low affinity, to estrogen receptors. It inhbits catechol-O-methyltransferase (COMT) activity. Inactivity of COMT blocks inactivation of catechol hormones and catecholamine neutransmitters. 2-Hydroxyestradiol is also reported to inhibit angiongensis and tumor cell growth (PMID: 9472688). [HMDB] |
|---|
| CAS Number | 362-05-0 |
|---|
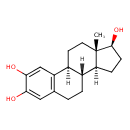
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (17b)-Estra-1,3, 5(10)-triene-2,3,17-triol | hmdb | | (17b)-Estra-1,3,5(10)-triene-2,3,17-triol | Generator | | (17beta)- Estra-1,3, 5 (10)-triene-2,3,17-triol | hmdb | | (17beta)-Estra-1,3,5(10)-triene-2,3,17-triol | hmdb | | (17β)-estra-1,3,5(10)-triene-2,3,17-triol | Generator | | 1,3,5(10)-estratriene-2,3,17Beta-triol | hmdb | | 17beta-2-Hydroxyestradiol | hmdb | | 2-hydroxy-17beta-estradiol | hmdb | | 2-hydroxy-estradiol | hmdb | | 2-Hydroxyestradiol | hmdb | | 2-Hydroxyestradiol-17b | Generator | | 2-Hydroxyestradiol-17beta | ChEBI | | 2-Hydroxyestradiol-17β | Generator | | 2-OH-e2 | ChEBI | | 2-OH-Estradiol | hmdb | | 2,3,17b-Trihydroxyestra-1,3,5(10)-triene | hmdb | | ECS | hmdb | | Estra-1,3,5 (10)-triene-2,3,17.beta.-triol | hmdb | | Estra-1,3,5(10)-triene-2,3,17-beta-triol | hmdb | | estra-1,3,5(10)-triene-2,3,17-triol (ACD/Name 4.0) | hmdb | | Estra-1,3,5(10)-triene-2,3,17b-triol | hmdb | | estra-1,3,5(10)-triene-2,3,17beta-triol | hmdb |
|
|---|
| Chemical Formula | C18H24O3 |
|---|
| IUPAC name | (1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-triene-4,5,14-triol |
|---|
| InChI Identifier | InChI=1S/C18H24O3/c1-18-7-6-11-12(14(18)4-5-17(18)21)3-2-10-8-15(19)16(20)9-13(10)11/h8-9,11-12,14,17,19-21H,2-7H2,1H3/t11-,12+,14-,17-,18-/m0/s1 |
|---|
| InChI Key | DILDHNKDVHLEQB-XSSYPUMDSA-N |
|---|
| Isomeric SMILES | [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])C3=C(CC[C@@]21[H])C=C(O)C(O)=C3 |
|---|
| Average Molecular Weight | 288.3814 |
|---|
| Monoisotopic Molecular Weight | 288.172544634 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrogens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Estrogen-skeleton
- 3-hydroxysteroid
- 2-hydroxysteroid
- Hydroxysteroid
- 17-hydroxysteroid
- Phenanthrene
- Tetralin
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Cyclic alcohol
- Secondary alcohol
- Polyol
- Organooxygen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 5 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 1354AH |
|---|
| Cayman Chemical | 13019 |
|---|
| Toronto Research Chemicals | H941890 |
|---|