| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:30:48 UTC |
|---|
| Update date | 2017-01-19 02:36:24 UTC |
|---|
| FoodComEx ID | PC000459 |
|---|
| FoodDB Record | FDB021921 |
|---|
| Chemical Information |
|---|
| Name | Thiosulfate |
|---|
| Description | Thiosulfate occurs naturally in hot springs and geysers, and is produced by certain biochemical processes. In the body, thiosulfate converts small amounts of cyanide ion into harmless products and plays a role in the biosynthesis of cysteine, a sulfur-containing amino acid that locks proteins into their correct three-dimensional shapes. Thiosulfate is not found in large quantities in nature. Solutions of thiosulfate break down into sulfur, sulfites, and sulfates when exposed to acids, light, metal ions, and bacteria. Thiosulfate is sometimes used as an antidote for cyanide poisoning. It reacts with cyanide to produce sulfite and thiocyanate ions: CN- + S2O32- SCN- + SO32- This reaction is catalyzed by an enzyme produced by cell mitochondria to neutralize small quantities of ingested cyanide (which occurs naturally in cassava root, lima beans, and almonds!). Thiosulfate is an intermediate in several biochemical pathways, including the synthesis of L-cysteine. Thiosulfate is manufactured by some cells by oxidation of elemental sulfur and by degradation of L-cysteine. Use: Photography (fixing agent to dissolve unchanged silver salts from exposed negatives), chrome tanning, removing chlorine in bleaching and papermaking, extraction of silver from its ores, dechlorination of water, mordant, reagent, bleaching, reducing agent in chrome dyeing, sequestrant in salt (up to 0.1%), antidote for cyanide poisoning. (Hawley's Condensed Chemical Dictionary) Source/Synthesis: Synthesis by dehydration of the pentahydrate at 105 degree. Alternatively formed by reaction of S2Cl2 with Na2O2 or by reduction of Na2S2O4 with sodium amalgam Use/Importance: Commercially available Biological Use/Importance: Cyanide antidote often administered with other antidotes, antifungal agent (ChemNetBase) Sodium thiosulfate is a common analytical reagent used in iodometric titration to analyze chlorine, bromine, and sulfide. Other uses are in bleaching paper pulp, bleaching straw, ivory, and bones, for removing chlorine from solutions, silver extraction from its ores, a mordant in dyeing and printing textiles, and as an antidote to cyanide poisoning. Another major application is in photography, where it is used as a fixer to dissolve unchanged silver salts from exposed negatives. (Handbook of Inorganic Chemicals) [HMDB] |
|---|
| CAS Number | 14383-50-7 |
|---|
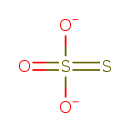
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| [SO3S](2-) | ChEBI | | Hyposulfite | hmdb | | Hyposulphite | Generator | | S-Hydril | hmdb | | S2O3(2-) | ChEBI | | Sodium thiosulfate | HMDB | | Sodium thiosulphate anhydrous | HMDB | | TETRATHIONATE | ChEBI | | TETRATHIONic acid | Generator | | Thiosulfate | hmdb | | Thiosulfate ion | hmdb | | Thiosulfate ion(2-) | ChEBI | | Thiosulfic acid | hmdb | | Thiosulfuric acid | hmdb | | Thiosulfuric acid (H2S2O3) | HMDB | | Thiosulfuric acid ion(2-) | Generator | | Thiosulphate | hmdb | | Thiosulphate ion | HMDB | | Thiosulphate ion(2-) | Generator | | Thiosulphuric acid | Generator | | Thiosulphuric acid ion(2-) | Generator | | Trioxido-1kappa(3)O-disulfate(S--S)(2-) | ChEBI | | Trioxido-1kappa(3)O-disulfuric acid(S--S)(2-) | Generator | | Trioxido-1kappa(3)O-disulphate(S--S)(2-) | Generator | | Trioxido-1kappa(3)O-disulphuric acid(S--S)(2-) | Generator |
|
|---|
| Chemical Formula | O3S2 |
|---|
| IUPAC name | sulfanidesulfonate |
|---|
| InChI Identifier | InChI=1S/H2O3S2/c1-5(2,3)4/h(H2,1,2,3,4)/p-2 |
|---|
| InChI Key | DHCDFWKWKRSZHF-UHFFFAOYSA-L |
|---|
| Isomeric SMILES | [O-]S([S-])(=O)=O |
|---|
| Average Molecular Weight | 112.128 |
|---|
| Monoisotopic Molecular Weight | 111.928885246 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of inorganic compounds known as non-metal thiosulfates. These are inorganic non-metallic compounds containing a thiosulfate as its largest oxoanion. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Homogeneous non-metal compounds |
|---|
| Class | Non-metal oxoanionic compounds |
|---|
| Sub Class | Non-metal thiosulfates |
|---|
| Direct Parent | Non-metal thiosulfates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Non-metal thiosulfate
- Inorganic oxide
- Inorganic sulfide
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |