| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:30:48 UTC |
|---|
| Update date | 2017-01-19 02:36:24 UTC |
|---|
| FoodComEx ID | PC000456 |
|---|
| FoodDB Record | FDB022215 |
|---|
| Chemical Information |
|---|
| Name | Lactulose |
|---|
| Description | A synthetic disaccharide used in the treatment of constipation and hepatic encephalopathy. It has also been used in the diagnosis of gastrointestinal disorders. (From Martindale, The Extra Pharmacopoeia, 30th ed, p887) [HMDB] |
|---|
| CAS Number | 4618-18-2 |
|---|
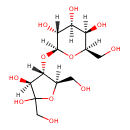
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2S,3R,4S,5R,6R)-2-[(2R,3S,4S,5R)-4,5-Dihydroxy-2,5-bis(hydroxymethyl)oxolan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol | HMDB | | 4-O-b-D-galactopyranosyl-D-Fructofuranose | hmdb | | 4-O-b-D-Galactopyranosyl-D-fructose | hmdb | | 4-O-beta-D-galactopyranosyl-D-Fructofuranose | hmdb | | 4-O-beta-D-Galactopyranosyl-D-fructose | hmdb | | 4-O-beta-delta-galactopyranosyl-delta-Fructofuranose | hmdb | | 4-O-beta-delta-Galactopyranosyl-delta-fructose | hmdb | | 4-O-β-D-galactopyranosyl-D-fructofuranose | Generator | | 4-O-β-D-galactopyranosyl-D-fructose | Generator | | Bifiteral | hmdb | | Cephulac | hmdb | | D-Lactulose | hmdb | | delta-Lactulose | hmdb | | Lactulosa | ChEBI | | Lactulose | hmdb | | Lactulosum | ChEBI |
|
|---|
| Chemical Formula | C12H22O11 |
|---|
| IUPAC name | (2S,3R,4S,5R,6R)-2-{[(2R,3S,4S)-4,5-dihydroxy-2,5-bis(hydroxymethyl)oxolan-3-yl]oxy}-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| InChI Identifier | InChI=1S/C12H22O11/c13-1-4-6(16)7(17)8(18)11(21-4)22-9-5(2-14)23-12(20,3-15)10(9)19/h4-11,13-20H,1-3H2/t4-,5-,6+,7+,8-,9-,10+,11+,12?/m1/s1 |
|---|
| InChI Key | JCQLYHFGKNRPGE-DNMRROERSA-N |
|---|
| Isomeric SMILES | [H][C@]1(CO)OC(O)(CO)[C@@]([H])(O)[C@]1([H])O[C@]1([H])O[C@]([H])(CO)[C@]([H])(O)[C@]([H])(O)[C@@]1([H])O |
|---|
| Average Molecular Weight | 342.2965 |
|---|
| Monoisotopic Molecular Weight | 342.116211546 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as o-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a O-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | O-glycosyl compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - O-glycosyl compound
- Disaccharide
- C-glycosyl compound
- Oxane
- Tetrahydrofuran
- Secondary alcohol
- Hemiacetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Acetal
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 2506AH |
|---|
| AKSci | J93965 |
|---|
| Cayman Chemical | 23795 |
|---|
| Glentham | GC1952 |
|---|
| MetaSci | HMDB0000740 |
|---|
| Sigma-Aldrich | HMDB0000740 |
|---|
| Toronto Research Chemicals | L165500 |
|---|