| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:30:47 UTC |
|---|
| Update date | 2017-01-19 02:36:24 UTC |
|---|
| FoodComEx ID | PC000452 |
|---|
| FoodDB Record | FDB021872 |
|---|
| Chemical Information |
|---|
| Name | Cortexolone |
|---|
| Description | Cortexolone is the precursor of cortisol. Accumulation of Cortexolone can happen in a defect known as congenital adrenal hyperplasia, which is due to 11-beta-hydroxylase deficiency, resulting in androgen excess, virilization, and hypertension. (PMID: 2022736)
A 17-hydroxycorticosteroid with glucocorticoid and anti-inflammatory activities. (PubChem) [HMDB] |
|---|
| CAS Number | 152-58-9 |
|---|
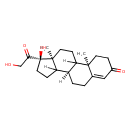
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 11-deoxy-17-hydroxy-Corticosterone | hmdb | | 11-Deoxy-17-hydroxycorticosterone | hmdb | | 11-deoxy-Cortisol | hmdb | | 11-Deoxycortisol | hmdb | | 11-Deoxyhydrocortisone | hmdb | | 11-Desoxy-17-hydroxycorticosterone | hmdb | | 11-Desoxycortisol | hmdb | | 11-Desoxyhydrocortisone | hmdb | | 11-dioxy-Cortisol | hmdb | | 11-Dioxycortisol | hmdb | | 17-Hydroxy-11-deoxycorticosterone | hmdb | | 17,21-Dihydroxy-4-pregnene-3,20-dione | hmdb | | 17,21-dihydroxypregn-4-ene-3,20-dione | hmdb | | 17,21-Dihydroxyprogesterone | hmdb | | 17alpha-Hydroxycortexone | hmdb | | 20-dione 17,21-Dihydroxypregn-4-ene-3 | hmdb | | 4-Pregnene-17alpha,21-diol-3,20-dione | hmdb | | Cortodoxone | hmdb | | Reichstein S | hmdb | | Reichstein's compound S | hmdb | | Reichstein's substance S | hmdb |
|
|---|
| Chemical Formula | C21H30O4 |
|---|
| IUPAC name | (2R,10R,14R,15S)-14-hydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one |
|---|
| InChI Identifier | InChI=1S/C21H30O4/c1-19-8-5-14(23)11-13(19)3-4-15-16(19)6-9-20(2)17(15)7-10-21(20,25)18(24)12-22/h11,15-17,22,25H,3-10,12H2,1-2H3/t15-,16?,17?,19+,20+,21+/m1/s1 |
|---|
| InChI Key | WHBHBVVOGNECLV-HMGFGKNBSA-N |
|---|
| Isomeric SMILES | [H]C12CC[C@](O)(C(=O)CO)[C@@]1(C)CCC1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |
|---|
| Average Molecular Weight | 346.4605 |
|---|
| Monoisotopic Molecular Weight | 346.214409448 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Hydroxysteroids |
|---|
| Direct Parent | 21-hydroxysteroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 21-hydroxysteroid
- Progestogin-skeleton
- Pregnane-skeleton
- 20-oxosteroid
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 17-hydroxysteroid
- Oxosteroid
- Delta-4-steroid
- Cyclohexenone
- Alpha-hydroxy ketone
- Tertiary alcohol
- Cyclic alcohol
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Organooxygen compound
- Primary alcohol
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 200 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| MetaSci | HMDB0000015 |
|---|
| Tokyo Chemical Industry | HMDB0000015 |
|---|
| Toronto Research Chemicals | D232600 |
|---|