| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:30:45 UTC |
|---|
| Update date | 2017-01-19 02:36:24 UTC |
|---|
| FoodComEx ID | PC000440 |
|---|
| FoodDB Record | FDB022702 |
|---|
| Chemical Information |
|---|
| Name | Aminopterin |
|---|
| Description | Aminopterin Syndrome Sine Aminopterin (ASSA, OMIM 600325) is an embryopathy caused by maternal treatment with the olic acid antagonist aminopterin has been recognized since 1952 when aminopterin was used as an abortifacient. The characteristic phenotype of the children who survived infancy after having been exposed to aminopterin or its methyl derivative, methotrexate, in early pregnancy included a very unusual facies, skull anomalies, and skeletal defects.(OMIM);
Aminopterin is an antimetabolite drug used in treatment of cancer and autoimmune diseases. It acts by inhibiting the metabolism of folic acid. - Wikipedia. The effects of the drug on intracellular metabolic processes, due to the inhibitory action on the enzyme dihydrofolate reductase, show that the result of this inhibition is more complex and is not limited to blockade of the reduction of folic acid alone. Although rescue methods are important in prevention of lethal effects of methotrexate, some metabolic pathways are insufficiently rescued, resulting in toxic reactions following methotrexate administration.(PMID 6398629) [HMDB] |
|---|
| CAS Number | 54-62-6 |
|---|
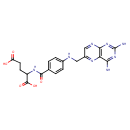
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 4-Amino-4-deoxypteroylglutamate | hmdb | | 4-Amino-PGA | hmdb | | 4-Aminofolate | hmdb | | 4-Aminofolic acid | hmdb | | 4-Aminopteroylglutamate | hmdb | | 4-Aminopteroylglutamic acid | hmdb | | Aminopterin | hmdb | | Aminopterine | hmdb | | APGA | hmdb | | L-N-[p-[[(2,4-diamino-6-pteridinyl)methyl]amino]benzoyl]-Glutamic acid | hmdb | | Pteramina | hmdb |
|
|---|
| Chemical Formula | C19H20N8O5 |
|---|
| IUPAC name | 2-[(4-{[(2,4-diaminopteridin-6-yl)methyl]amino}phenyl)formamido]pentanedioic acid |
|---|
| InChI Identifier | InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27) |
|---|
| InChI Key | TVZGACDUOSZQKY-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NC1=NC2=C(N=C(CNC3=CC=C(C=C3)C(=O)NC(CCC(O)=O)C(O)=O)C=N2)C(N)=N1 |
|---|
| Average Molecular Weight | 440.4127 |
|---|
| Monoisotopic Molecular Weight | 440.15566579 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as folic acids. These are heterocyclic compounds based on the 4-[(pteridin-6-ylmethyl)amino]benzoic acid skeleton conjugated with one or more L-glutamate units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Folic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Folic acid
- Glutamic acid or derivatives
- Hippuric acid or derivatives
- Hippuric acid
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid or derivatives
- Aminobenzamide
- Aminobenzoic acid or derivatives
- Benzamide
- Benzoic acid or derivatives
- Benzoyl
- Phenylalkylamine
- Aniline or substituted anilines
- Aminopyrimidine
- Aralkylamine
- Secondary aliphatic/aromatic amine
- Pyrimidine
- Pyrazine
- Monocyclic benzene moiety
- Benzenoid
- Imidolactam
- Dicarboxylic acid or derivatives
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Amino acid
- Carboxamide group
- Secondary amine
- Azacycle
- Carboxylic acid derivative
- Carboxylic acid
- Hydrocarbon derivative
- Amine
- Organonitrogen compound
- Organic oxide
- Organic nitrogen compound
- Organopnictogen compound
- Carbonyl group
- Primary amine
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 200 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | Z3082 |
|---|
| Cayman Chemical | 21802 |
|---|
| Glentham | GK2974 |
|---|
| Toronto Research Chemicals | A628850 |
|---|