| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:30:35 UTC |
|---|
| Update date | 2017-01-19 02:36:22 UTC |
|---|
| FoodComEx ID | PC000411 |
|---|
| FoodDB Record | FDB001205 |
|---|
| Chemical Information |
|---|
| Name | Galactaric acid |
|---|
| Description | Present in ripe fruits of peach and pear. Formed in grapes and grape must by the action of Botrytis cinerea on galacturonic acid
Galactaric acid is the dicarboxylic sugar acid resulting from oxidation glactose with dilute nitric acid. It is a substrate of galactarate O-hydroxycinnamoyltransferase [EC 2.3.1.130]. (KEGG); It forms a crystalline powder which melts at 213 °C. It is insoluble in alcohol, and nearly insoluble in cold water. Due to the symmetry in the molecule, it is optically inactive even though it has chiral carbon atoms (i.e., it is a meso compound). When heated with pyridine to 140 °C, it is converted into allommic acid. When digested with fuming hydrochloric acid for some time it is converted into a 2,5-furandicarboxylic acid (FDCA) while on heating with barium sulfide it is transformed into athiophene carboxylic acid. The ammonium salt yields on dry distillation carbon dioxide, ammonia, pyrrol and other substances. The acid when fused with caustic alkalis yields oxalic acid. Galactaric acid is found in many foods, some of which are alcoholic beverages, fruits, pomes, and peach. |
|---|
| CAS Number | 526-99-8 |
|---|
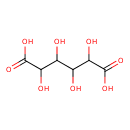
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2R,3S,4R,5S)-2,3,4,5-tetrahydroxyhexanedioate | biospider | | (2R,3S,4R,5S)-2,3,4,5-tetrahydroxyhexanedioic acid | biospider | | 1,2,3,4-Tetrahydroxy-1,4-butanedicarboxylic acid | db_source | | Acido galactarico | biospider | | Acido mucico | biospider | | D-Galactaric acid | HMDB | | Galactarate | Generator | | Galactarsaeure | ChEBI | | Galactosaccharate | biospider | | Galactosaccharic acid | db_source | | Galaktarsaeure | ChEBI | | Hexaric acid | biospider | | Meso-galactaric acid | biospider | | MTPA | biospider | | Mucate | Generator | | Mucic acid | db_source | | Mucinsaeure | ChEBI | | Saccharolactate | biospider | | Saccharolactic acid | ChEBI | | Schleimsaeure | ChEBI | | Schleimsaure | HMDB | | Tetrahydroxyadipic acid | db_source | | Tetrahydroxyhexanedioic acid | biospider |
|
|---|
| Chemical Formula | C6H10O8 |
|---|
| IUPAC name | 2,3,4,5-tetrahydroxyhexanedioic acid |
|---|
| InChI Identifier | InChI=1S/C6H10O8/c7-1(3(9)5(11)12)2(8)4(10)6(13)14/h1-4,7-10H,(H,11,12)(H,13,14) |
|---|
| InChI Key | DSLZVSRJTYRBFB-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OC(C(O)C(O)C(O)=O)C(O)C(O)=O |
|---|
| Average Molecular Weight | 210.1388 |
|---|
| Monoisotopic Molecular Weight | 210.037567296 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as glucuronic acid derivatives. Glucuronic acid derivatives are compounds containing a glucuronic acid moiety (or a derivative), which consists of a glucose moiety with the C6 carbon oxidized to a carboxylic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Glucuronic acid derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glucuronic acid or derivatives
- Medium-chain hydroxy acid
- Medium-chain fatty acid
- Beta-hydroxy acid
- Hydroxy fatty acid
- Monosaccharide
- Hydroxy acid
- Dicarboxylic acid or derivatives
- Alpha-hydroxy acid
- Fatty acyl
- Fatty acid
- Secondary alcohol
- Polyol
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | 3.3 mg/mL at 14 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | Mp 230° dec. | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | V1455 |
|---|
| MetaSci | HMDB0000639 |
|---|
| Sigma-Aldrich | HMDB0000639 |
|---|
| Toronto Research Chemicals | G155005 |
|---|