| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:30:30 UTC |
|---|
| Update date | 2017-01-19 02:36:22 UTC |
|---|
| FoodComEx ID | PC000408 |
|---|
| FoodDB Record | FDB021809 |
|---|
| Chemical Information |
|---|
| Name | Cytidine |
|---|
| Description | Cytidine is a nucleoside that is composed of the base cytosine linked to the five-carbon sugar D-ribose. Cytidine is a pyrimidine that besides being incorporated into nucleic acids, can serve as substrate for the salvage pathway of pyrimidine nucleotide synthesis; as precursor of the cytidine triphosphate (CTP) needed in the phosphatidylcholine (PC) and phosphatidylethanolamine (PE) biosynthetic pathway. These variations probably reflect the species differences in cytidine deaminase, the enzyme that converts cytidine to uridine in the body. The transports of cytidine into the brain's extracellular fluid, and then into neurons and glia, are essential prerequisites for cytidine to be utilized in brain. An efficient mechanism mediating the brain uptake of circulating cytidine has not yet been demonstrated. The biosynthesis of PC, the most abundant phosphatide in the brain, via the Kennedy pathway requires phosphocholine and cytidine triphosphate (CTP), a cytidine nucleotide, which is involved in the rate-limiting step. The enzyme that converts CTP to endogenous CDP-choline (CTP: phosphocholine cytidylyltransferase) is unsaturated at physiological brain CTP levels.
APOBEC is a family of enzymes has been discovered with the ability to deaminate cytidines on RNA or DNA. The human apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G (APOBEC3G, or hA3G) protein, provides cells with an intracellular antiretroviral activity that is associated with the hypermutation of viral DNA through cytidine deamination. Indeed, hA3G belongs to a family of vertebrate proteins that contain one or two copies of a signature sequence motif unique to cytidine deaminases (CTDAs). (PMID: 16769123, 15780864, 16720547) [HMDB]. Cytidine is found in many foods, some of which are sweet potato, rocket salad (sspecies), mexican groundcherry, and cinnamon. |
|---|
| CAS Number | 65-46-3 |
|---|
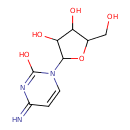
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1-(b-D-Ribofuranosyl)-2-oxo-4-amino-1,2-dihydro-1,3-diazine | HMDB | | 1-(b-delta-Ribofuranosyl)-2-oxo-4-amino-1,2-dihydro-1,3-diazine | HMDB | | 1-b-D-Ribofuranosylcytosine | Generator | | 1-b-D-Ribosyl-cytosine | HMDB | | 1-beta-D-Ribofuranosyl-cytosine | HMDB | | 1-beta-D-Ribofuranosylcytosine | ChEBI | | 1-beta-delta-Ribofuranosyl-cytosine | HMDB | | 1-beta-delta-Ribofuranosylcytosine | HMDB | | 1-beta-delta-Ribosyl-cytosine | HMDB | | 1-β-D-ribofuranosylcytosine | Generator | | 1b-D-Ribofuranosylcytosine | Generator | | 1beta-D-Ribofuranosylcytosine | ChEBI | | 1beta-delta-Ribofuranosylcytosine | HMDB | | 1beta-Ribofuranosylcytosine | HMDB | | 1β-D-ribofuranosylcytosine | Generator | | 4-amino-1-b-D-RIBOFURANOSYL-2(1H)-pyrimidinone | Generator | | 4-amino-1-b-D-Ribofuranosylpyrimidin-2(1H)-one | Generator | | 4-amino-1-BETA-D-RIBOFURANOSYL-2(1H)-pyrimidinone | ChEBI | | 4-amino-1-beta-D-Ribofuranosylpyrimidin-2(1H)-one | ChEBI | | 4-amino-1-beta-delta-Ribofuranosyl-2(1H)-pyrimidinone | HMDB | | 4-amino-1-β-D-ribofuranosyl-2(1H)-pyrimidinone | Generator | | 4-amino-1-β-D-ribofuranosylpyrimidin-2(1H)-one | Generator | | 4-amino-1b-D-Ribofuranosyl-2(1H)-pyrimidinone | Generator | | 4-amino-1beta-D-Ribofuranosyl-2(1H)-pyrimidinone | ChEBI | | 4-amino-1β-D-ribofuranosyl-2(1H)-pyrimidinone | Generator | | Cyd | ChEBI | | Cytidin | ChEBI | | Cytosine riboside | ChEBI | | Cytosine-1b-D-ribofuranoside | Generator | | Cytosine-1b-delta-ribofuranoside | HMDB | | Cytosine-1beta-D-ribofuranoside | ChEBI | | Cytosine-1beta-delta-ribofuranoside | HMDB | | Cytosine-1β-D-ribofuranoside | Generator | | Zytidin | ChEBI |
|
|---|
| Chemical Formula | C9H13N3O5 |
|---|
| IUPAC name | 4-amino-1-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,2-dihydropyrimidin-2-one |
|---|
| InChI Identifier | InChI=1S/C9H13N3O5/c10-5-1-2-12(9(16)11-5)8-7(15)6(14)4(3-13)17-8/h1-2,4,6-8,13-15H,3H2,(H2,10,11,16) |
|---|
| InChI Key | UHDGCWIWMRVCDJ-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OCC1OC(C(O)C1O)N1C=CC(=N)N=C1O |
|---|
| Average Molecular Weight | 243.2166 |
|---|
| Monoisotopic Molecular Weight | 243.085520541 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as pyrimidine nucleosides. Pyrimidine nucleosides are compounds comprising a pyrimidine base attached to a ribosyl or deoxyribosyl moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Pyrimidine nucleosides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Pyrimidine nucleosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidine nucleoside
- Glycosyl compound
- N-glycosyl compound
- Pentose monosaccharide
- Aminopyrimidine
- Pyrimidone
- Hydropyrimidine
- Monosaccharide
- Pyrimidine
- Imidolactam
- Tetrahydrofuran
- Heteroaromatic compound
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Organic nitrogen compound
- Primary alcohol
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Organopnictogen compound
- Organic oxygen compound
- Primary amine
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 50 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | F964 |
|---|
| AKSci | J90237 |
|---|
| Glentham | GC1136 |
|---|
| MetaSci | HMDB0000089 |
|---|
| Sigma-Aldrich | HMDB0000089 |
|---|
| Toronto Research Chemicals | C998300 |
|---|