| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:30:26 UTC |
|---|
| Update date | 2017-01-19 02:36:22 UTC |
|---|
| FoodComEx ID | PC000396 |
|---|
| FoodDB Record | FDB022060 |
|---|
| Chemical Information |
|---|
| Name | Biopterin |
|---|
| Description | Biopterin concentrations in cerebrospinal fluid from patients with Parkinson's disease, in which the nigrostriatal dopamine neurons degenerate, are lower than those from age-matched older controls. In hereditary progressive dystonia/DOPA-responsive dystonia, which is a dopamine deficiency caused by mutations in GTP cyclohydrolase I without neuronal cell death (Segawa's disease), biopterin in cerebrospinal fluid decrease in parallel owing to the decreased activity in GTP cyclohydrolase I (EC 3.5.4.16, is an enzyme that is part of the folate and biopterin biosynthesis pathways. It is responsible for the hydrolysis of guanosine triphosphate (GTP) to form 7,8-dihydroneopterin 3'-triphosphate. (Pteridines (1999), 10(1), 5-13.) Lowered levels of urinary biopterin concomitant with elevated serum phenylalanine concentration occur in a variant type of hyperphenylalaninemia caused by a deficiency of tetrahydrobiopterin (BH4), the obligatory cofactor for phenylalanine hydroxylase. The most frequent form of this cofactor deficiency is due to lack of 6-pyruvoyl-tetrahydropterin synthase (PTPS) activity, the second enzyme in the biosynthetic pathway for BH4. (PMID 8178819) The hepatic phenylalanine hydroxylating system consists of 3 essential components, phenylalanine hydroxylase, dihydropteridine reductase, and the nonprotein coenzyme, tetrahydrobiopterin. The reductase and the pterin coenzyme are also essential components of the tyrosine and tryptophan hydroxylating systems. There are 3 distinct forms of phenylketonuria or hyperphenylalaninemia, each caused by lack of 1 of these essential components. The variant forms of the disease that are caused by the lack of dihydropteridine reductase or tetrahydrobiopterin are characterized by severe neurol. deterioration, impaired functioning of tyrosine and tryptophan hydroxylases, and the resultant deficiency of tyrosine- and tryptophan-derived monoamine neurotransmitters in brain. (PMID 3930837) [HMDB] |
|---|
| CAS Number | 22150-76-1 |
|---|
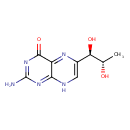
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (-)-Biopterin | hmdb | | (1'R,1'S) Biopterin | hmdb | | [S-(R*,S*)]-2-amino-6-(1,2-dihydroxypropyl)-4(1H)-Pteridinone | hmdb | | 2-amino-6-(L-erythro-1,2-dihydroxypropyl)-4(3H)-Pteridinone | hmdb | | 6-Biopterin | hmdb | | Biopterin | hmdb | | L-Biopterin | hmdb | | L-erythro-Biopterin | hmdb | | Pterin H B2 | hmdb |
|
|---|
| Chemical Formula | C9H11N5O3 |
|---|
| IUPAC name | 2-amino-6-[(1R,2S)-1,2-dihydroxypropyl]-4,8-dihydropteridin-4-one |
|---|
| InChI Identifier | InChI=1S/C9H11N5O3/c1-3(15)6(16)4-2-11-7-5(12-4)8(17)14-9(10)13-7/h2-3,6,15-16H,1H3,(H3,10,11,13,14,17)/t3-,6-/m0/s1 |
|---|
| InChI Key | LHQIJBMDNUYRAM-DZSWIPIPSA-N |
|---|
| Isomeric SMILES | C[C@H](O)[C@H](O)C1=NC2=C(NC(N)=NC2=O)N=C1 |
|---|
| Average Molecular Weight | 237.2153 |
|---|
| Monoisotopic Molecular Weight | 237.086189243 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as biopterins and derivatives. These are coenzymes containing a 2-amino-pteridine-4-one derivative. They are mainly synthesized in several parts of the body, including the pineal gland. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Biopterins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Biopterin
- Aminopyrimidine
- Pyrimidone
- Pyrazine
- Pyrimidine
- Heteroaromatic compound
- Vinylogous amide
- 1,2-diol
- Secondary alcohol
- Azacycle
- Organic oxygen compound
- Alcohol
- Aromatic alcohol
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organic oxide
- Amine
- Organopnictogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 10 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | V0321 |
|---|
| Cayman Chemical | 10007662 |
|---|
| Toronto Research Chemicals | B389020 |
|---|