| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:30:25 UTC |
|---|
| Update date | 2017-01-19 02:36:22 UTC |
|---|
| FoodComEx ID | PC000393 |
|---|
| FoodDB Record | FDB021897 |
|---|
| Chemical Information |
|---|
| Name | beta-D-Glucuronic acid |
|---|
| Description | Glucuronic acid is a carboxylic acid that has the structure of a glucose molecule that has had its sixth carbon atom (of six total) oxidized. The salts of glucuronic acid are known as glucuronates. Glucuronic acid is highly soluble in water. In the animal body, glucuronic acid is often linked to poisonous substances to allow for subsequent elimination, and to hormones to allow for easier transport. These linkages involve O-glycosidic bonds. The process is known as glucuronidation, and the resulting substances are known as glucuronides (or glucuronosides). Glucuronidation uses UDP-glucuronic acid (glucuronic acid linked via a glycosidic bond to uridine diphosphate) as an intermediate. UDP-glucuronic acid is formed in the liver of all animals. [HMDB]
Widely distributed in plants, where it occurs in gums, mucilages, saponins and flavone glycosides and in animals as a constituent of mucopolysaccharides. Glycosides are formed in the liver to detoxify poisonous hydroxyl-containing substances. Phenyl, cresyl and indoxyl glycosides are present in normal urine. [CCD]. beta-D-Glucuronic acid is found in many foods, some of which are cashew nut, american cranberry, sour cherry, and soy bean. |
|---|
| CAS Number | 6556-12-3 |
|---|
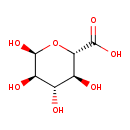
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| alpha-D-Glucopyranuronic acid | HMDB | | alpha-D-Glucuronic acid | HMDB | | alpha-delta-Glucopyranuronic acid | HMDB | | alpha-delta-Glucuronic acid | HMDB | | D-(+)-Glucuronate | hmdb | | D-(+)-Glucuronic acid | hmdb | | D-Glucuronate | hmdb | | D-GLUCURONIC ACID | ChEBI | | delta-(+)-Glucuronate | HMDB | | delta-(+)-Glucuronic acid | HMDB | | delta-Glucuronate | HMDB | | GCU | HMDB | | GlcAa | ChEBI | | GlcAalpha | ChEBI | | Glucosiduronate | hmdb | | Glucosiduronic acid | hmdb | | Glucuronate | hmdb | | Glucuronic acid | hmdb |
|
|---|
| Chemical Formula | C6H10O7 |
|---|
| IUPAC name | (2S,3S,4S,5R,6S)-3,4,5,6-tetrahydroxyoxane-2-carboxylic acid |
|---|
| InChI Identifier | InChI=1S/C6H10O7/c7-1-2(8)4(5(10)11)13-6(12)3(1)9/h1-4,6-9,12H,(H,10,11)/t1-,2-,3+,4-,6-/m0/s1 |
|---|
| InChI Key | AEMOLEFTQBMNLQ-WAXACMCWSA-N |
|---|
| Isomeric SMILES | O[C@H]1O[C@@H]([C@@H](O)[C@H](O)[C@H]1O)C(O)=O |
|---|
| Average Molecular Weight | 194.1394 |
|---|
| Monoisotopic Molecular Weight | 194.042652674 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as glucuronic acid derivatives. Glucuronic acid derivatives are compounds containing a glucuronic acid moiety (or a derivative), which consists of a glucose moiety with the C6 carbon oxidized to a carboxylic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Glucuronic acid derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glucuronic acid or derivatives
- Beta-hydroxy acid
- Hydroxy acid
- Pyran
- Monosaccharide
- Oxane
- Hemiacetal
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Oxacycle
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Polyol
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Glentham | GC9013 |
|---|
| MetaSci | HMDB0000127 |
|---|
| Tokyo Chemical Industry | HMDB0000127 |
|---|
| Toronto Research Chemicals | G596850 |
|---|