| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:30:22 UTC |
|---|
| Update date | 2017-01-19 02:36:21 UTC |
|---|
| FoodComEx ID | PC000379 |
|---|
| FoodDB Record | FDB022125 |
|---|
| Chemical Information |
|---|
| Name | 5beta-Coprostanol |
|---|
| Description | Coprosterol or coprostanol is a cholesterol derivative found in human feces, gallstones, eggs, and other biological matter. Coprosterol is the odorous principle of feces. It is formed from the biohydrogenation of cholesterol (cholest-5en-3β-ol) in the gut of most higher animals and birds. This compound has frequently been used as a biomarker for the presence of human faecal matter in the environment. American physician Austin Flint named it stercorin . The transformation of cholesterol into coprosterol in its passage through the body involves a reduction of the C5:C6 double bond, and a transition from the allocholanic- to the cholanic-ring system. Although it is established that the bacterial flora of the intestine is concerned in the reduction process, the mechanism by which the stereochemical change is brought about is unknown. Current data suggests that cholestenone and coprostanone, and not cholesterol itself, are the immediate precursors of coprosterol which is formed from them in the intestine by bacterial reduction. [HMDB] |
|---|
| CAS Number | 360-68-9 |
|---|
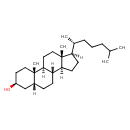
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (3b,5b)-cholestan-3-ol | hmdb | | 3b-Hydroxy-5b-cholestanol | hmdb | | 5b-Cholestan-3b-ol | hmdb | | 5b-Coprostanol | hmdb | | 5beta Coprostanol | hmdb | | 5beta-cholestan-3beta-ol | hmdb | | 5beta-Coprostanol | hmdb | | Stercorin | hmdb |
|
|---|
| Chemical Formula | C27H48O |
|---|
| IUPAC name | (1S,2S,5S,7R,10R,11S,14R,15R)-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-5-ol |
|---|
| InChI Identifier | InChI=1S/C27H48O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h18-25,28H,6-17H2,1-5H3/t19-,20-,21+,22+,23-,24+,25+,26+,27-/m1/s1 |
|---|
| InChI Key | QYIXCDOBOSTCEI-NWKZBHTNSA-N |
|---|
| Isomeric SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@]4([H])C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |
|---|
| Average Molecular Weight | 388.6694 |
|---|
| Monoisotopic Molecular Weight | 388.370516158 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as cholesterols and derivatives. Cholesterols and derivatives are compounds containing a 3-hydroxylated cholestane core. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Cholestane steroids |
|---|
| Direct Parent | Cholesterols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cholesterol-skeleton
- Cholesterol
- 3-beta-hydroxysteroid
- Hydroxysteroid
- 3-hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 5 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Toronto Research Chemicals | C685450 |
|---|