| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:30:16 UTC |
|---|
| Update date | 2017-01-19 02:36:21 UTC |
|---|
| FoodComEx ID | PC000371 |
|---|
| FoodDB Record | FDB022181 |
|---|
| Chemical Information |
|---|
| Name | L-Kynurenine |
|---|
| Description | Kynurenine is a metabolite of the amino acid tryptophan used in the production of niacin.
L-kynurenine is a central compound of the pathway of tryptophan metabolism pathway since it can change to the neuroprotective agent kynurenic acid or to the neurotoxic agent quinolinic acid. The break-up of these endogenous compounds' balance can be observable in many disorders. It can occur in neurodegenerative disorders, such as Parkinson's disease, Huntington's and Alzheimer's disease, in stroke, in epilepsy, in multiple sclerosis, in amyotrophic lateral sclerosis, and in mental failures, such as schizophrenia and depression. [HMDB] |
|---|
| CAS Number | 2922-83-0 |
|---|
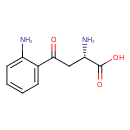
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2S)-2-amino-4-(2-Aminophenyl)-4-oxobutanoate | Generator | | (2S)-2-amino-4-(2-Aminophenyl)-4-oxobutanoic acid | ChEBI | | (alphaS)-alpha,2-diamino-3-hydroxy-gamma-oxo-Benzenebutanoate | hmdb | | (alphaS)-alpha,2-diamino-3-hydroxy-gamma-oxo-Benzenebutanoic acid | hmdb | | (S)-alpha,2-diamino-3-hydroxy-gamma-oxo-Benzenebutanoate | hmdb | | (S)-alpha,2-diamino-3-hydroxy-gamma-oxo-Benzenebutanoic acid | hmdb | | 3-(3-Hydroxyanthraniloyl)-L-alanine | HMDB | | 3-anthraniloyl-Alanine | hmdb | | 3-anthraniloyl-L-alanine | hmdb | | 3-Anthraniloylalanine | hmdb | | 3-Hydroxy-L-kynurenine | HMDB | | alpha,2-diamino-gamma-oxo-Benzenebutanoate | hmdb | | alpha,2-diamino-gamma-oxo-Benzenebutanoic acid | hmdb | | dl-Kynurenine | hmdb | | DL-Kynureninefree base | hmdb | | Kynurenin | hmdb | | KYNURENINE | ChEBI | | Quinurenine | hmdb |
|
|---|
| Chemical Formula | C10H12N2O3 |
|---|
| IUPAC name | (2S)-2-amino-4-(2-aminophenyl)-4-oxobutanoic acid |
|---|
| InChI Identifier | InChI=1S/C10H12N2O3/c11-7-4-2-1-3-6(7)9(13)5-8(12)10(14)15/h1-4,8H,5,11-12H2,(H,14,15)/t8-/m0/s1 |
|---|
| InChI Key | YGPSJZOEDVAXAB-QMMMGPOBSA-N |
|---|
| Isomeric SMILES | N[C@@H](CC(=O)C1=CC=CC=C1N)C(O)=O |
|---|
| Average Molecular Weight | 208.2139 |
|---|
| Monoisotopic Molecular Weight | 208.08479226 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alkyl-phenylketones. These are aromatic compounds containing a ketone substituted by one alkyl group, and a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Alkyl-phenylketones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkyl-phenylketone
- Butyrophenone
- Alpha-amino acid
- Alpha-amino acid or derivatives
- L-alpha-amino acid
- Benzoyl
- Gamma-keto acid
- Aniline or substituted anilines
- Aryl alkyl ketone
- Monocyclic benzene moiety
- Beta-aminoketone
- Keto acid
- Benzenoid
- Vinylogous amide
- Amino acid
- Amino acid or derivatives
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Primary aliphatic amine
- Amine
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxide
- Organic nitrogen compound
- Organonitrogen compound
- Primary amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | E641 |
|---|
| Cayman Chemical | 11305 |
|---|
| MetaSci | HMDB0000684 |
|---|
| Sigma-Aldrich | HMDB0000684 |
|---|
| Toronto Research Chemicals | K661005 |
|---|