| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:30:04 UTC |
|---|
| Update date | 2017-01-19 02:36:20 UTC |
|---|
| FoodComEx ID | PC000343 |
|---|
| FoodDB Record | FDB022021 |
|---|
| Chemical Information |
|---|
| Name | 2-Methoxyestradiol |
|---|
| Description | 2-Methoxyestradiol (2ME2) is a drug that prevents the formation of new blood vessels that tumors need in order to grow (angiogenesis). It is derived from estrogen, although it binds poorly to known estrogen receptors, and belongs to the family of drugs called angiogenesis inhibitors. It has undergone Phase 1 clinical trials against breast cancers. Preclinical models also suggest that 2ME2 could also be effective against inflammatory diseases such as rheumatoid arthritis. The CAS name for 2ME2 is (17 beta)-2-methoxyestra-1,3,5(10)-triene-3,17-diol. It also acts as a vasodilator. [HMDB] |
|---|
| CAS Number | 362-07-2 |
|---|
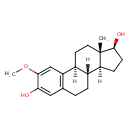
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1,3,5(10)-ESTRATRIEN-2,3,17-b-triol 2-methyl ether | Generator | | 1,3,5(10)-ESTRATRIEN-2,3,17-BETA-triol 2-methyl ether | ChEBI | | 1,3,5(10)-ESTRATRIEN-2,3,17-β-triol 2-methyl ether | Generator | | 2-Hydroxyestradol 2-methyl ether | ChEBI | | 2-Methoxyestradiol | hmdb | | 2-Methoxyestradiol-17b | Generator | | 2-Methoxyestradiol-17beta | ChEBI | | 2-Methoxyestradiol-17β | Generator | | Panzem | ChEBI |
|
|---|
| Chemical Formula | C19H26O3 |
|---|
| IUPAC name | (1S,10R,11S,14S,15S)-4-methoxy-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-triene-5,14-diol |
|---|
| InChI Identifier | InChI=1S/C19H26O3/c1-19-8-7-12-13(15(19)5-6-18(19)21)4-3-11-9-16(20)17(22-2)10-14(11)12/h9-10,12-13,15,18,20-21H,3-8H2,1-2H3/t12-,13+,15-,18-,19-/m0/s1 |
|---|
| InChI Key | CQOQDQWUFQDJMK-SSTWWWIQSA-N |
|---|
| Isomeric SMILES | [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])C3=C(CC[C@@]21[H])C=C(O)C(OC)=C3 |
|---|
| Average Molecular Weight | 302.4079 |
|---|
| Monoisotopic Molecular Weight | 302.188194698 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrogens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Estrogen-skeleton
- 3-hydroxysteroid
- 17-hydroxysteroid
- Hydroxysteroid
- Phenanthrene
- Tetralin
- Anisole
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Benzenoid
- Cyclic alcohol
- Secondary alcohol
- Ether
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Alcohol
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 20 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | X5011 |
|---|
| Cayman Chemical | 13021 |
|---|
| Toronto Research Chemicals | M262625 |
|---|