| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:30:01 UTC |
|---|
| Update date | 2017-01-19 02:36:20 UTC |
|---|
| FoodComEx ID | PC000338 |
|---|
| FoodDB Record | FDB021881 |
|---|
| Chemical Information |
|---|
| Name | Androsterone |
|---|
| Description | Androsterone is an inactive breakdown metabolite of testosterone, the product of a reaction mediated by the enzyme oxidative 17beta-hydroxysteroid dehydrogenase (EC 1.1.1.51, 17beta-HSD). Androsterone is also can be metabolized from other adrenal androgens such as dehydroepiandrosterone, dihydrotestosterone or androstenedione, and is considered an inactive end product; however, it can be a physiological effector in its own right. Androsterone might be converted back to dihydrotestosterone. Humans (and other primates) are unique among mammals in having high levels of circulating androsterone glucuronide, a process that is the major role uridine-diphospho-glucuronosyltransferase (EC 2.4.1.17, UGT) enzymes for glucuronidation of steroid metabolism in humans. Conjugation of androsterone is a pathway found in all vertebrates and is widely recognized that the liver is a major site of glucuronidation; however it is now clear that extrahepatic tissues are also involved in the conjugation of compounds to which these tissues are exposed. High levels of androsterone glucuronide found in the human prostate, breast cyst fluid and ovary follicular fluid suggest that glucuronidation of 5alpha-reduced C19 steroids occurs in these tissues as well. In doping control, the ratio of androsterone/etiocholanone provides valuable information that allows the assignment of a urine specimen to a particular person or the identification of urine samples with identical steroid profiles; this is particularly important to detect attempts of urine manipulation including urine alteration and substitution. (PMID: 9188497, 17017935, 14643063, 12943709, 9699884, 17260133) [HMDB] |
|---|
| CAS Number | 53-41-8 |
|---|
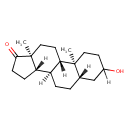
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (3alpha,5alpha)-3-hydroxy-Androstan-17-one | hmdb | | (3R,5S,8R,9S,10S,13S,14S)-3-hydroxy-10,13-dimethyl-1,2,3,4,5,6,7,8,9,11,12,14,15,16-tetradecahydrocyclopenta[a]phenanthren-17-one | hmdb | | 3-alpha-Hydroxy-17-androstanone | hmdb | | 3-alpha-Hydroxy-5-alpha-androstan-17-one | hmdb | | 3-alpha-Hydroxy-5-alpha-androstane-17-one | hmdb | | 3-alpha-hydroxy-5alpha-Androstan-17-one | hmdb | | 3-alpha-Hydroxyetioallocholan-17-one | hmdb | | 3-Epihydroxyetioallocholan-17-one | hmdb | | 3-hydroxy-(3-alpha,5-alpha)-Androstan-17-one | hmdb | | 3-Hydroxyandrostan-17-one | hmdb | | 3a-Hydroxyetioallocholan-17-one | hmdb | | 3alpha-Hydroxy-17-androstanone | hmdb | | 3alpha-Hydroxy-5alpha-androstan-17-one | hmdb | | 3alpha-Hydroxyetioallocholan-17-one | hmdb | | 5-alpha-Androstan-3-alpha-ol-17-one | hmdb | | 5-alpha-Androstane-3alpha-ol-17-one | hmdb | | 5-alpha-Androsterone | hmdb | | 5a-Androstan-3a-ol-17-one | hmdb | | 5a-Androstane-3a-ol-17-one | hmdb | | 5a-Androsterone | hmdb | | 5alpha-Androstane-3alpha-ol-17-one | hmdb | | 5alpha-Androsterone | hmdb | | Androkinine | hmdb | | Androstanon-3-alpha-ol-17-one | hmdb | | Androsterone | hmdb | | Androtine | hmdb | | Atromide ICI | hmdb | | cis-Androsterone | hmdb |
|
|---|
| Chemical Formula | C19H30O2 |
|---|
| IUPAC name | (1S,2S,7S,10R,11S,15S)-5-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-one |
|---|
| InChI Identifier | InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12-16,20H,3-11H2,1-2H3/t12-,13?,14-,15-,16-,18-,19-/m0/s1 |
|---|
| InChI Key | QGXBDMJGAMFCBF-XYQQMQERSA-N |
|---|
| Isomeric SMILES | [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(O)CC[C@]12C |
|---|
| Average Molecular Weight | 290.4403 |
|---|
| Monoisotopic Molecular Weight | 290.224580204 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Androstane steroids |
|---|
| Direct Parent | Androgens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Androgen-skeleton
- Oxosteroid
- 17-oxosteroid
- Hydroxysteroid
- 3-hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 100 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | H126 |
|---|
| AKSci | J10414 |
|---|
| AKSci | HMDB0000031 |
|---|
| Cayman Chemical | 15872 |
|---|
| MetaSci | HMDB0000031 |
|---|
| Toronto Research Chemicals | A637535 |
|---|
| Toronto Research Chemicals | KIT1055 |
|---|