| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:59 UTC |
|---|
| Update date | 2017-01-19 02:36:20 UTC |
|---|
| FoodComEx ID | PC000335 |
|---|
| FoodDB Record | FDB014510 |
|---|
| Chemical Information |
|---|
| Name | Biotin |
|---|
| Description | Present in many foods; particularly rich sources include yeast, eggs, liver, certain fish (e.g. mackerel, salmon, sardines), soybeans, cauliflower and cow peas. Dietary supplement. Isolated from various higher plant sources, e.g. sweet corn seedlings and radish leaves
Biotin D(+) is a cofactor responsible for carbon dioxide transfer in several carboxylase enzymes:; Biotin binds very tightly to the tetrameric protein avidin (also streptavidin and neutravidin), with a dissociation constant Kd in the order of 10?15 mol/L which is one of the strongest known protein-ligand interactions, approaching the covalent bond in strength. This is often used in different biotechnological applications. Until 2005, very harsh conditions were required to break the biotin-streptavidin bond.; Biotin is a water-soluble B-complex vitamin which is composed of an ureido ring fused with a tetrahydrothiophene ring. A valeric acid substituent is attached to one of the carbon atoms of the tetrahydrothiophene ring. Biotin is used in cell growth, the production of fatty acids, metabolism of fats, and amino acids. It plays a role in the Kreb cycle, which is the process in which energy is released from food. Biotin not only assists in various metabolic chemical conversions, but also helps with the transfer of carbon dioxide. Biotin is also helpful in maintaining a steady blood sugar level. Biotin is often recommended for strengthening hair and nails. Consequenty, it is found in many cosmetic and health products for the hair and skin. Biotin deficiency is a rare nutritional disorder caused by a deficiency of biotin. Initial symptoms of biotin deficiency include: Dry skin, Seborrheic dermatitis, Fungal infections, rashes including erythematous periorofacial macular rash, fine and brittle hair, and hair loss or total alopecia. If left untreated, neurological symptoms can develop, including mild depression, which may progress to profound lassitude and, eventually, to somnolence; Biotin is an enzyme co-factor present in minute amounts in every living cell. Biotin is also known as vitamin H or B7 or coenzyme R. It occurs mainly bound to proteins or polypeptides and is abundant in liver, kidney, pancreas, yeast, and milk. Biotin has been recognized as an essential nutrient. Our biotin requirement is fulfilled in part through diet, through endogenous reutilization of biotin and perhaps through capture of biotin generated in the intestinal flora. The utilization of biotin for covalent attachment to carboxylases and its reutilization through the release of carboxylase biotin after proteolytic degradation constitutes the 'biotin cycle'. Biotin deficiency is associated with neurological manifestations, skin rash, hair loss and metabolic disturbances that are thought to relate to the various carboxylase deficiencies (metabolic ketoacidosis with lactic acidosis). It has also been suggested that biotin deficiency is associated with protein malnutrition, and that marginal biotin deficiency in pregnant women may be teratogenic. Biotin acts as a carboxyl carrier in carboxylation reactions. There are four biotin-dependent carboxylases in mammals: those of propionyl-CoA (PCC), 3-methylcrotonyl-CoA (MCC), pyruvate (PC) and acetyl-CoA carboxylases (isoforms ACC-1 and ACC-2). All but ACC-2 are mitochondrial enzymes. The biotin moiety is covalently bound to the epsilon amino group of a Lysine residue in each of these carboxylases in a domain 60-80 amino acids long. The domain is structurally similar among carboxylases from bacteria to mammals. There are four biotin-dependent carboxylases in mammals: those of propionyl-CoA (PCC), 3-methylcrotonyl-CoA (MCC), pyruvate (PC) and acetyl-CoA carboxylases (isoforms ACC-1 and ACC-2). All but ACC-2 are mitochondrial enzymes. The biotin moiety is covalently bound to the epsilon amino group of a Lys residue in each of these carboxylases in a domain 60-80 amino acids long. The domain is structurally similar among carboxylases from bacteria to mammals. Evidence is emerging that biotin participates in processes other than classical carboxylation reactions. Specifically, novel roles for biotin in cell signaling, gene expression, and chromatin structure have been identified in recent years. Human cells accumulate biotin by using both the sodium-dependent multivitamin transporter and monocarboxylate transporter 1. These transporters and other biotin-binding proteins partition biotin to compartments involved in biotin signaling: cytoplasm, mitochondria, and nuclei. The activity of cell signals such as biotinyl-AMP, Sp1 and Sp3, nuclear factor (NF)-kappaB, and receptor tyrosine kinases depends on biotin supply. Consistent with a role for biotin and its catabolites in modulating these cell signals, greater than 2000 biotin-dependent genes have been identified in various human tissues. Many biotin-dependent gene products play roles in signal transduction and localize to the cell nucleus, consistent with a role for biotin in cell signaling. Posttranscriptional events related to ribosomal activity and protein folding may further contribute to effects of biotin on gene expression. Finally, research has shown that biotinidase and holocarboxylase synthetase mediate covalent binding of biotin to histones (DNA-binding proteins), affecting chromatin structure; Biotin, also known as vitamin H or B7, is a water-soluble B-complex vitamin which is composed of an ureido (tetrahydroimidizalone) ring fused with a tetrahydrothiophene ring. A valeric acid substituent is attached to one of the carbon atoms of the tetrahydrothiophene ring. Biotin is a cofactor in the metabolism of fatty acids and leucine, and it plays a role in gluconeogenesis.; Signs of Biotin Deficiency: In general, appetite and growth are decreased. Dermatologic symptoms include dermatitis, alopecia (hair loss) and achromotrichia (absence or loss of pigment in the hair.) Perosis (a shortening and thickening of bones) is seen in the skeleton. Fatty Liver and Kidney Syndrome (FLKS) and hepatic steatosis also can occur. Genetic defect could also cause biotin deficiency. Holocarboxylase synthetase deficiency is a genetic mutation. It is a severe metabolic disorder. Biochemical and clinical manifestation includes: ketolactic acidosis, organic aciduria, hyperammonemia, skin rash, feeding problems, hypotonie, seizures, development delay, alopecia, and coma. This disease is lethal, however, mentioned manifestation can be reversed by pharmacologic doses of biotin (10-100 mg per day).[citation needed]; at least seven biotinylation sites have been identified in human histones. Biotinylation of histones appears to play a role in cell proliferation, gene silencing, and the cellular response to DNA repair. Roles for biotin in cell signaling and chromatin structure are consistent with the notion that biotin has a unique significance in cell biology. (PMID: 15992684, 16011464) |
|---|
| CAS Number | 58-85-5 |
|---|
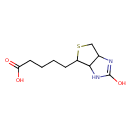
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| -(+)-biotin | biospider | | (+)-biotin | biospider | | (+)-cis-Hexahydro-2-oxo-1H-thieno[3,4]imidazole-4-valerate | biospider | | (+)-cis-hexahydro-2-oxo-1H-thieno[3,4]Imidazole-4-valeric acid | ChEBI | | (3AS,4S,6ar)-hexahydro-2-oxo-1H-thieno[3,4-D]imidazole-4-valerate | Generator | | (3AS,4S,6ar)-hexahydro-2-oxo-1H-thieno[3,4-D]imidazole-4-valeric acid | ChEBI | | 1avd | biospider | | 1ndj | biospider | | 1stp | biospider | | 1swg | biospider | | 1swk | biospider | | 1swn | biospider | | 1swp | biospider | | 1swr | biospider | | 2avi | biospider | | 3H-Biotin | biospider | | 5-(2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanoate | biospider | | 5-(2-Oxohexahydro-1H-thieno[3,4-D]imidazol-4-yl)pentanoic acid | ChEBI | | Beta-biotin | biospider | | Biodermatin | biospider | | Bioepiderm | biospider | | Bioepiderm (TN) | biospider | | Bios h | biospider | | Bios II | biospider | | Biotin (8CI) | biospider | | Biotin (jan/usp/inn) | biospider | | Biotin [usan:inn:jan] | biospider | | Biotin 50 Mcg | biospider | | Biotin111In | biospider | | Biotina | ChEBI | | Biotine | ChEBI | | Biotinum | biospider | | BTN | biospider | | cis-(+)-Tetrahydro-2-oxothieno[3,4]imidazoline-4-valerate | biospider | | cis-(+)-Tetrahydro-2-oxothieno[3,4]imidazoline-4-valeric acid | ChEBI | | cis-hexahydro-2-oxo-1H-thieno(3,4)Imidazole-4-valerate | Generator | | cis-Hexahydro-2-oxo-1H-thieno(3,4)imidazole-4-valeric acid | biospider | | cis-Tetrahydro-2-oxothieno(3,4-D)imidazoline-4-valerate | Generator | | cis-Tetrahydro-2-oxothieno(3,4-d)imidazoline-4-valeric acid | biospider | | Coenzyme R | db_source | | D-(+)-biotin | biospider | | D-biotin | biospider | | D-biotin factor s | biospider | | D(+)-biotin | biospider | | Delta-(+)-biotin | biospider | | Delta-biotin | biospider | | Delta-biotin factor s | biospider | | Factor s | biospider | | Factor s (vitamin) | biospider | | Factor S? | db_source | | hexahydro-2-oxo-[3AS-(3aa,4b,6aa)]-1H-thieno[3,4-D]imidazole-4-pentanoate | HMDB | | hexahydro-2-oxo-[3AS-(3aa,4b,6aa)]-1H-thieno[3,4-D]imidazole-4-pentanoic acid | HMDB | | hexahydro-2-oxo-[3As-(3alpha,4beta,6alpha)]-1H-thieno[3,4-D]imidazole-4-pentanoate | HMDB | | hexahydro-2-oxo-[3As-(3alpha,4beta,6alpha)]-1H-thieno[3,4-D]imidazole-4-pentanoic acid | HMDB | | Hexahydro-2-oxo-1H-thieno(3,4-d)imidazole-4-pentanoate | biospider | | Hexahydro-2-oxo-1H-thieno(3,4-d)imidazole-4-pentanoic acid | biospider | | Hexahydro-2-oxo-1H-thieno[3,4-d]imidazole-4-pentanoic acid, 9CI | db_source | | Injacom h | biospider | | Lutavit H2 | biospider | | Medebiotin | biospider | | Meribin | biospider | | Ritatin | biospider | | Rovimix H 2 | biospider | | Vitamin B7 | db_source | | Vitamin BW | biospider | | Vitamin H | db_source | | Vitamin h (van) | biospider | | Vitamin-h | biospider |
|
|---|
| Chemical Formula | C10H16N2O3S |
|---|
| IUPAC name | 5-{2-hydroxy-1H,3aH,4H,6H,6aH-thieno[3,4-d]imidazol-6-yl}pentanoic acid |
|---|
| InChI Identifier | InChI=1S/C10H16N2O3S/c13-8(14)4-2-1-3-7-9-6(5-16-7)11-10(15)12-9/h6-7,9H,1-5H2,(H,13,14)(H2,11,12,15) |
|---|
| InChI Key | YBJHBAHKTGYVGT-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OC(=O)CCCCC1SCC2NC(=O)NC12 |
|---|
| Average Molecular Weight | 244.311 |
|---|
| Monoisotopic Molecular Weight | 244.088163078 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as biotin and derivatives. These are organic compounds containing a ureido (tetrahydroimidizalone) ring fused with a tetrahydrothiophene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Biotin and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Biotin and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Biotin
- Imidazolyl carboxylic acid derivative
- Medium-chain fatty acid
- Heterocyclic fatty acid
- Thia fatty acid
- Fatty acid
- Fatty acyl
- Thiolane
- 2-imidazoline
- Isourea
- Azacycle
- Dialkylthioether
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Carboxylic acid derivative
- Thioether
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Organonitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | 0.22 mg/mL at 25 oC | MERCK INDEX (1996) |
|---|
| Melting Point | Mp 232-233° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 400 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | C820 |
|---|
| AKSci | J11016 |
|---|
| AKSci | J91293 |
|---|
| Cayman Chemical | 22582 |
|---|
| Glentham | GV4685 |
|---|
| MetaSci | HMDB0000030 |
|---|
| Sigma-Aldrich | HMDB0000030 |
|---|
| Toronto Research Chemicals | B389040 |
|---|