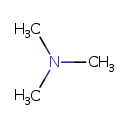| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:56 UTC |
|---|
| Update date | 2017-01-19 02:36:20 UTC |
|---|
| FoodComEx ID | PC000328 |
|---|
| FoodDB Record | FDB011944 |
|---|
| Chemical Information |
|---|
| Name | Trimethylamine |
|---|
| Description | Trimethylamine, also known as NMe3 or TMA, is a nitrogenous base and can be readily protonated to give trimethylammonium cation. Trimethylammonium chloride is a hygroscopic colorless solid prepared from hydrochloric acid. Trimethylamine is a product of decomposition of plants and animals. It is the substance mainly responsible for the fishy odor often associated with fouling fish, bacterial vagina infections, and bad breath. It is also associated with taking large doses of choline (Wikipedia). Trimethylamine is an organic compound with the formula N(CH3)3. This colorless, hygroscopic, and flammable tertiary amine has a strong "fishy" odor in low concentrations and an ammonia-like odor at higher concentrations. It is a gas at room temperature but is usually sold in pressurized gas cylinders or as a 40% solution in water. Trimethylamine has a boiling point of 2.9 degree centigrade. Trimethylamine is a nitrogenous base and its positively charged cation is called trimethylammonium cation. A common salt of trimethylamine is trimethylammonium chloride, a hygroscopic colorless solid (Wikipedia). Trimethylaminuria is a genetic disorder in which the body is unable to metabolize trimethylamine from food sources. Patients develop a characteristic fish odour of their sweat, urine, and breath after the consumption of choline-rich foods. Trimethylaminuria is an autosomal recessive disorder involving a trimethylamine oxidase deficiency. Trimethylaminuria has also been observed in a certain breed of Rhode Island Red chicken that produces eggs with a fishy smell (Wikipedia). Trimethylamine in the urine is a biomarker for the consumption of legumes. Trimethylamine is found in many foods, some of which are fishes, alcoholic beverages, milk and milk products, and rice. |
|---|
| CAS Number | 75-50-3 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (CH3)3N | ChEBI | | (CH3)3NH | biospider | | (CH3)3NH+ | biospider | | 2840-24-6 (hydrobromide) | biospider | | Dimethylmethaneamine | biospider | | FEMA 3241 | db_source | | HBR of trimethylamine | biospider | | HCL of trimethylamine | biospider | | Hi of trimethylamine | biospider | | Methanamine, n,n-dimethyl- | biospider | | Methylamine, n,n-dimethyl- | biospider | | N-trimethylamine | biospider | | N,n-dimethyl-methanamine | biospider | | N,n-dimethylmethanamine | biospider | | N,N-Dimethylmethanamine, 9CI | db_source | | N,N,N-Trimethylamine | ChEBI | | N(CH3)3 | ChEBI | | NMe3 | biospider | | TMA | biospider | | TML | biospider | | Tridimethylaminomethane | ChEBI | | Trimethylamin | biospider | | Trimethylamine anhydrous | biospider | | Trimethylamine aqueous solution | HMDB | | Trimethylamine-14C hydrochloride | biospider | | Trimethylamine, anhydrous [UN1083] [Flammable gas] | biospider | | Trimethylamine(alkyl-substituted derivatives) | biospider | | Trimethylammonium chloride | biospider |
|
|---|
| Chemical Formula | C3H9N |
|---|
| IUPAC name | trimethylamine |
|---|
| InChI Identifier | InChI=1S/C3H9N/c1-4(2)3/h1-3H3 |
|---|
| InChI Key | GETQZCLCWQTVFV-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CN(C)C |
|---|
| Average Molecular Weight | 59.1103 |
|---|
| Monoisotopic Molecular Weight | 59.073499293 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as trialkylamines. These are organic compounds containing a trialkylamine group, characterized by exactly three alkyl groups bonded to the amino nitrogen. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | Trialkylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tertiary aliphatic amine
- Organopnictogen compound
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | 0.16 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 890 mg/mL at 30 oC | SCHWEIZER,AE et al. (1978) |
|---|
| Melting Point | Mp -117.2° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Toronto Research Chemicals | T796175 |
|---|