| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:51 UTC |
|---|
| Update date | 2017-01-19 02:36:19 UTC |
|---|
| FoodComEx ID | PC000318 |
|---|
| FoodDB Record | FDB021884 |
|---|
| Chemical Information |
|---|
| Name | Dihydrobiopterin |
|---|
| Description | Dihydrobiopterin (BH2) is an oxidation product of tetrahydrobiopterin. Tetrahydrobiopterin is a natural occurring cofactor of the aromatic amino acid hydroxylase and is involved in the synthesis of tyrosine and the neurotransmitters dopamine and serotonin. Tetrahydrobiopterin is also essential for nitric oxide synthase catalyzed oxidation of L-arginine to L-citrulline and nitric oxide. [HMDB] |
|---|
| CAS Number | 6779-87-9 |
|---|
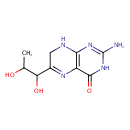
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (6R)-6-(L-erythro-1,2-Dihydroxypropyl)-7,8-dihydro-6H-pterin | hmdb | | (S-(R*,S*))-2-amino-6-(1,2-dihydroxypropyl)-7,8-dihydro-4(1H)-Pteridinone | hmdb | | 2-amino-6-((1R,2S)-1,2-dihydroxypropyl)-7,8-dihydro-4(1H)-Pteridinone | hmdb | | 2-amino-6-(1,2-dihydroxypropyl)-7,8-dihydro-4(1H)-Pteridinone | hmdb | | 6,7-Dihydrobiopterin | hmdb | | 7,8-Dihydro-L-biopterin | hmdb | | 7,8-Dihydrobiopterin | hmdb | | BH2 | hmdb | | Dihydrobiopterin | hmdb | | L-erythro-1-(2-amino-7,8-dihydro-4-hydroxy-6-pteridinyl)-1,2-Propanediol | hmdb | | L-erythro-7,8-Dihydrobiopterin | hmdb | | L-erythro-Dihydrobiopterin | hmdb | | L-erythro-q-Dihydrobiopterin | hmdb | | Quinoid-dihydrobiopterin | hmdb | | Quinonoid dihydro-(6H)-biopterin | ChEBI | | Quinonoid dihydrobiopterin | hmdb |
|
|---|
| Chemical Formula | C9H13N5O3 |
|---|
| IUPAC name | 2-amino-6-(1,2-dihydroxypropyl)-3,4,7,8-tetrahydropteridin-4-one |
|---|
| InChI Identifier | InChI=1S/C9H13N5O3/c1-3(15)6(16)4-2-11-7-5(12-4)8(17)14-9(10)13-7/h3,6,15-16H,2H2,1H3,(H4,10,11,13,14,17) |
|---|
| InChI Key | FEMXZDUTFRTWPE-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CC(O)C(O)C1=NC2=C(NC1)N=C(N)NC2=O |
|---|
| Average Molecular Weight | 239.2312 |
|---|
| Monoisotopic Molecular Weight | 239.101839307 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as biopterins and derivatives. These are coenzymes containing a 2-amino-pteridine-4-one derivative. They are mainly synthesized in several parts of the body, including the pineal gland. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Biopterins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Biopterin
- Secondary aliphatic/aromatic amine
- Hydroxypyrimidine
- Pyrimidine
- Heteroaromatic compound
- 1,2-diol
- Ketimine
- Secondary alcohol
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Secondary amine
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Imine
- Organic nitrogen compound
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 10 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 1494AH |
|---|
| Cayman Chemical | 81882 |
|---|
| Toronto Research Chemicals | D448550 |
|---|