| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:42 UTC |
|---|
| Update date | 2017-01-19 02:36:19 UTC |
|---|
| FoodComEx ID | PC000294 |
|---|
| FoodDB Record | FDB022699 |
|---|
| Chemical Information |
|---|
| Name | L-Norleucine |
|---|
| Description | An unnatural amino acid that is used experimentally to study protein structure and function. It is structurally similar to methionine, however it does not contain sulfur.
Aminocaproic acid works as an antifibrinolytic. It is a derivative of the amino acid lysine. It binds reversibly to the kringle domain of plasminogen and blocks the binding of plasminogen to fibrin and its activation to plasmin. [HMDB] |
|---|
| CAS Number | 327-57-1 |
|---|
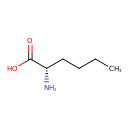
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (S)-2-amino-Hexanoate | hmdb | | (S)-2-amino-Hexanoic acid | hmdb | | (S)-2-Aminohexanoate | hmdb | | (S)-2-Aminohexanoic acid | hmdb | | (S)-Aminohexanoate | hmdb | | (S)-Aminohexanoic acid | hmdb | | (S)-Norleucine | hmdb | | 2-Aminocaproate | hmdb | | 2-Aminocaproic acid | hmdb | | 2-Aminohexanoate | hmdb | | 2-Aminohexanoic acid | hmdb | | a-Aminocaproate | Generator | | a-Aminocaproic acid | Generator | | alpha-Aminocaproate | Generator | | alpha-Aminocaproic acid | ChEBI | | Caprine | ChEBI | | Glycoleucine | hmdb | | L-(+)-Norleucine | hmdb | | L-2-Aminohexanoate | hmdb | | L-2-Aminohexanoic acid | hmdb | | L-Aminohexanoate | ChEBI | | L-Aminohexanoic acid | ChEBI | | L-Norleucine | hmdb | | L(+)-Norleucine | ChEBI | | Nle | ChEBI | | Norleucine | hmdb | | α-aminocaproate | Generator | | α-aminocaproic acid | Generator |
|
|---|
| Chemical Formula | C6H13NO2 |
|---|
| IUPAC name | (2S)-2-aminohexanoic acid |
|---|
| InChI Identifier | InChI=1S/C6H13NO2/c1-2-3-4-5(7)6(8)9/h5H,2-4,7H2,1H3,(H,8,9)/t5-/m0/s1 |
|---|
| InChI Key | LRQKBLKVPFOOQJ-YFKPBYRVSA-N |
|---|
| Isomeric SMILES | CCCC[C@H](N)C(O)=O |
|---|
| Average Molecular Weight | 131.1729 |
|---|
| Monoisotopic Molecular Weight | 131.094628665 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - L-alpha-amino acid
- Medium-chain fatty acid
- Amino fatty acid
- Fatty acid
- Fatty acyl
- Amino acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Organic nitrogen compound
- Amine
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 200 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | H414 |
|---|
| AKSci | J91157 |
|---|
| MetaSci | HMDB0001645 |
|---|
| Sigma-Aldrich | HMDB0001645 |
|---|
| Toronto Research Chemicals | N712000 |
|---|