| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:42 UTC |
|---|
| Update date | 2017-01-19 02:36:19 UTC |
|---|
| FoodComEx ID | PC000293 |
|---|
| FoodDB Record | FDB022701 |
|---|
| Chemical Information |
|---|
| Name | Ethenodeoxyadenosine |
|---|
| Description | Etheno (epsilon) modified DNA bases are generated from the carcinogens vinyl chloride and urethane, but also by reactions of DNA with products derived from lipid peroxidation (LPO) and oxidative stress via endogenous pathways. Highly variable background levels of epsilon-adducts were detected in DNA from different organs of unexposed humans and rodents. Several known cancer risk factors increased the level of these DNA lesions in target organs: elevated epsilon-adducts were found in hepatic DNA from patients with metal storage diseases, after overproduction of nitric oxide (NO) by inducible NO synthase (iNOS) in a mouse model, and in colonic polyps of familial adenomatous polyposis patients. A high omega-6-polyunsaturated fatty acid diet increased epsilon-DNA adducts in white blood cells of female subjects. (PMID: 10882861) [HMDB] |
|---|
| CAS Number | 68498-25-9 |
|---|
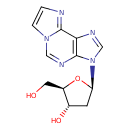
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1,N(6)-Ethenodeoxyadenosine | hmdb | | 1,N6-Etheno-2'-deoxyadenosine | hmdb | | 1,N6-Etheno-dA | hmdb | | 1,N6-Ethenodeoxyadenosine | hmdb | | Ethenodeoxyadenosine | hmdb | | N1,N6-Etheno-2'-deoxyadenosine | hmdb |
|
|---|
| Chemical Formula | C12H13N5O3 |
|---|
| IUPAC name | (2R,3S,5R)-2-(hydroxymethyl)-5-{3H-imidazo[2,1-f]purin-3-yl}oxolan-3-ol |
|---|
| InChI Identifier | InChI=1S/C12H13N5O3/c18-4-8-7(19)3-9(20-8)17-6-14-10-11-13-1-2-16(11)5-15-12(10)17/h1-2,5-9,18-19H,3-4H2/t7-,8+,9+/m0/s1 |
|---|
| InChI Key | XQQIMTUYVDUWKJ-DJLDLDEBSA-N |
|---|
| Isomeric SMILES | OC[C@H]1O[C@H](C[C@@H]1O)N1C=NC2=C1N=CN1C=CN=C21 |
|---|
| Average Molecular Weight | 275.2633 |
|---|
| Monoisotopic Molecular Weight | 275.101839307 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as purines and purine derivatives. These are aromatic heterocyclic compounds containing a purine moiety, which is formed a pyrimidine-ring ring fused to an imidazole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazopyrimidines |
|---|
| Sub Class | Purines and purine derivatives |
|---|
| Direct Parent | Purines and purine derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Purine
- N-substituted imidazole
- Pyrimidine
- Heteroaromatic compound
- Tetrahydrofuran
- Imidazole
- Azole
- Secondary alcohol
- Oxacycle
- Azacycle
- Hydrocarbon derivative
- Organic nitrogen compound
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 5 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 8628AJ |
|---|
| Glentham | GN5101 |
|---|
| Toronto Research Chemicals | E890380 |
|---|