| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:42 UTC |
|---|
| Update date | 2017-01-19 02:36:19 UTC |
|---|
| FoodComEx ID | PC000292 |
|---|
| FoodDB Record | FDB002281 |
|---|
| Chemical Information |
|---|
| Name | N-Acetyl-L-cysteine |
|---|
| Description | Effective inhibitor of enzymic browning in foods [DFC]
N-Acetyl-L-cysteine is a pharmaceutical drug and nutritional supplement used primarily as a mucolytic agent and in the management of paracetamol (acetaminophen) overdose. Other uses include sulfate repletion in conditions, such as autism, where cysteine and related sulfur amino acids may be depleted. N-Acetyl-L-cysteine is a precursor in the formation of the antioxidant glutathione in the body. The thiol group confers antioxidant effects and is able to reduce free radicals. [Wikipedia]. |
|---|
| CAS Number | 616-91-1 |
|---|
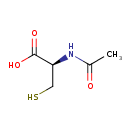
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2R)-2-(Acetylamino)-3-mercaptopropanoic acid | biospider | | (2R)-2-Acetamido-3-sulfanyl-propanoic acid | biospider | | (2R)-2-acetylamino-3-Sulfanylpropanoate | Generator | | (2R)-2-Acetylamino-3-sulfanylpropanoic acid | biospider | | (2R)-2-acetylamino-3-Sulphanylpropanoate | Generator | | (2R)-2-acetylamino-3-Sulphanylpropanoic acid | Generator | | (R)-2-acetylamino-3-Mercaptopropanoate | Generator | | (R)-2-Acetylamino-3-mercaptopropanoic acid | biospider | | (R)-Mercaptate | Generator | | (R)-Mercaptic acid | Generator | | (R)-Mercapturic acid | ChEBI | | 2-Acetylamino-3-mercapto-propionate | biospider | | 2-Acetylamino-3-mercapto-propionic acid | biospider | | Acetadote | biospider | | Acetein | biospider | | Acetilcisteina | ChEBI | | Acetylcysteine | manual | | Acetylcysteinum | ChEBI | | Airbron | db_source | | Fabrol | db_source | | Fluimicil infantil | HMDB | | Fluimucetin | HMDB | | Flumucetin | HMDB | | Fluprowit | HMDB | | L-2-Acetamido-3-mercaptopropanoic acid | manual | | L-a-acetamido-b-Mercaptopropionate | Generator | | L-a-acetamido-b-Mercaptopropionic acid | Generator | | L-Acetylcysteine | manual | | L-alpha-acetamido-beta-Mercaptopropionate | Generator | | L-alpha-Acetamido-beta-mercaptopropionic acid | biospider | | L-α-acetamido-β-mercaptopropionate | Generator | | L-α-acetamido-β-mercaptopropionic acid | Generator | | Lysomucil | biospider | | Mercaptate | Generator | | Mercaptic acid | Generator | | Mercapturic acid | ChEBI | | Mucomyst | db_source | | N-Acety-L-cysteine | HMDB | | N-Acetyl cysteine | biospider | | N-Acetyl-3-mercaptoalanine | biospider | | N-Acetyl-L-(+)-cysteine | biospider | | N-Acetyl-L-cysteine | biospider | | N-Acetylcysteine | biospider | | N-Acetylcysteine; L-form | db_source | | NAC | biospider | | NSC 111180 | db_source | | Parvolex | db_source | | Respaire | db_source | | Sodium 2-acetamido-3-mercaptopropionate | HMDB |
|
|---|
| Chemical Formula | C5H9NO3S |
|---|
| IUPAC name | (2R)-2-acetamido-3-sulfanylpropanoic acid |
|---|
| InChI Identifier | InChI=1S/C5H9NO3S/c1-3(7)6-4(2-10)5(8)9/h4,10H,2H2,1H3,(H,6,7)(H,8,9)/t4-/m0/s1 |
|---|
| InChI Key | PWKSKIMOESPYIA-BYPYZUCNSA-N |
|---|
| Isomeric SMILES | CC(=O)N[C@@H](CS)C(O)=O |
|---|
| Average Molecular Weight | 163.195 |
|---|
| Monoisotopic Molecular Weight | 163.030313849 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as n-acyl-l-alpha-amino acids. These are n-acylated alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | N-acyl-L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-l-alpha-amino acid
- Cysteine or derivatives
- Acetamide
- Carboxamide group
- Secondary carboxylic acid amide
- Alkylthiol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Hydrocarbon derivative
- Organic oxygen compound
- Organic nitrogen compound
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Organopnictogen compound
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 109-110° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | I630 |
|---|
| AKSci | J90138 |
|---|
| Cayman Chemical | 20261 |
|---|
| Fluka | HMDB0001890 |
|---|
| Glentham | GM8803 |
|---|
| MetaSci | HMDB0001890 |
|---|
| Toronto Research Chemicals | A172091 |
|---|