| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:39 UTC |
|---|
| Update date | 2017-01-19 02:36:19 UTC |
|---|
| FoodComEx ID | PC000285 |
|---|
| FoodDB Record | FDB021820 |
|---|
| Chemical Information |
|---|
| Name | Pyridoxal 5'-phosphate |
|---|
| Description | Pyridoxal 5'-phosphate, also known as PLP or codecarboxylase, belongs to the class of organic compounds known as pyridoxals and derivatives. Pyridoxals and derivatives are compounds containing a pyridoxal moiety, which consists of a pyridine ring substituted at positions 2,3,4, and 5 by a methyl group, a hydroxyl group, a carbaldehyde group, and a hydroxymethyl group, respectively. Pyridoxal 5'-phosphate is a drug which is used for nutritional supplementation and for treating dietary shortage or imbalance. Pyridoxal 5'-phosphate is a strong basic compound (based on its pKa). Pyridoxal 5'-phosphate exists in all living species, ranging from bacteria to humans. In humans, pyridoxal 5'-phosphate is involved in glycine and serine metabolism. Outside of the human body, Pyridoxal 5'-phosphate is found, on average, in the highest concentration within milk (cow). Pyridoxal 5'-phosphate has also been detected, but not quantified in, several different foods, such as soursops, italian sweet red peppers, muscadine grapes, european plums, and blackcurrants. This could make pyridoxal 5'-phosphate a potential biomarker for the consumption of these foods. The monophosphate ester obtained by condensation of phosphoric acid with the primary hydroxy group of pyridoxal. Pyridoxal 5'-phosphate is a potentially toxic compound. Pyridoxal 5'-phosphate, with regard to humans, has been found to be associated with several diseases such as epilepsy, early-onset, vitamin b6-dependent, odontohypophosphatasia, pyridoxamine 5-prime-phosphate oxidase deficiency, and hypophosphatasia; pyridoxal 5'-phosphate has also been linked to the inborn metabolic disorder celiac disease. |
|---|
| CAS Number | 54-47-7 |
|---|
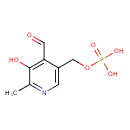
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 3-Hydroxy-2-methyl-5-[(onooxy)methyl]-4-pyridinecarboxaldehyde | ChEBI | | 3-Hydroxy-5-(hydroxymethyl)-2-methylisonicotinaldehyde 5-ate | ChEBI | | 3-Hydroxy-5-(hydroxymethyl)-2-methylisonicotinaldehyde 5-ic acid | Generator | | Apolon b6 | HMDB | | Biosechs | HMDB | | Codecarboxylase | ChEBI | | Coenzyme b6 | HMDB | | Hairoxal | HMDB | | Hexermin-P | HMDB | | Hi-pyridoxin | HMDB | | Hiadelon | HMDB | | Himitan | HMDB | | Opyridoxal | HMDB | | Opyridoxal coenzyme | HMDB | | Orate mono-(4-formyl-5-hydroxy-6-methyl-pyridin-3-ylmethyl) ester | Generator | | oric acid Mono-(4-formyl-5-hydroxy-6-methyl-pyridin-3-ylmethyl) ester | ChEBI | | PAL-P | HMDB | | Pidopidon | HMDB | | Piodel | HMDB | | PLP | ChEBI | | Pydoxal | HMDB | | Pyridoxal 5-monoate ester | Generator | | Pyridoxal 5-monoic acid ester | Generator | | Pyridoxal 5-monooric acid ester | ChEBI | | Pyridoxal 5-ate | ChEBI | | Pyridoxal 5-ic acid | Generator | | Pyridoxal 5'-(dihydrogen ate) | ChEBI | | Pyridoxal 5'-(dihydrogen ic acid) | Generator | | Pyridoxal 5'-ate | ChEBI | | Pyridoxal 5'-ic acid | Generator | | Pyridoxal ate | ChEBI | | Pyridoxal ic acid | Generator | | Pyridoxal P | HMDB | | PYRIDOXAL-5'-ATE | ChEBI | | PYRIDOXAL-5'-ic acid | Generator | | Pyridoxal-P | HMDB | | Pyridoxyl ate | HMDB | | Pyromijin | HMDB | | Sechvitan | HMDB | | Vitahexin-P | HMDB | | Vitazechs | HMDB |
|
|---|
| Chemical Formula | C8H10NO6P |
|---|
| IUPAC name | [(4-formyl-5-hydroxy-6-methylpyridin-3-yl)methoxy]phosphonic acid |
|---|
| InChI Identifier | InChI=1S/C8H10NO6P/c1-5-8(11)7(3-10)6(2-9-5)4-15-16(12,13)14/h2-3,11H,4H2,1H3,(H2,12,13,14) |
|---|
| InChI Key | NGVDGCNFYWLIFO-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CC1=NC=C(COP(O)(O)=O)C(C=O)=C1O |
|---|
| Average Molecular Weight | 247.1419 |
|---|
| Monoisotopic Molecular Weight | 247.024573569 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as pyridoxals and derivatives. Pyridoxals and derivatives are compounds containing a pyridoxal moiety, which consists of a pyridine ring substituted at positions 2,3,4, and 5 by a methyl group, a hydroxyl group, a carbaldehyde group, and a hydroxymethyl group, respectively. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pyridine carboxaldehydes |
|---|
| Direct Parent | Pyridoxals and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyridoxal
- Aryl-aldehyde
- Monoalkyl phosphate
- Hydroxypyridine
- Methylpyridine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Vinylogous acid
- Heteroaromatic compound
- Azacycle
- Organopnictogen compound
- Aldehyde
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Toronto Research Chemicals | P991715 |
|---|