| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:36 UTC |
|---|
| Update date | 2017-01-19 02:36:18 UTC |
|---|
| FoodComEx ID | PC000278 |
|---|
| FoodDB Record | FDB021885 |
|---|
| Chemical Information |
|---|
| Name | Argininosuccinic acid |
|---|
| Description | Arginosuccinic acid is a basic amino acid. Some cells synthesize it from citrulline, aspartic acid and use it as a precursor for arginine in the urea cycle or Citrulline-NO cycle. The enzyme that catalyzes the reaction is argininosuccinate synthetase. Argininosuccinic acid is a precursor to fumarate in the citric acid cycle via argininosuccinate lyase. Defects in the arginosuccinate lyase enzyme can lead to arginosuccinate lyase deficiency. Argininosuccinate (ASA) lyase deficiency results in defective cleavage of ASA. This leads to an accumulation of ASA in cells and an excessive excretion of ASA in urine (arginosuccinic aciduria). In virtually all respects, this disorder shares the characteristics of other urea cycle defects. The most important characteristic of ASA lyase deficiency is its propensity to cause hyperammonemia in affected individuals. ASA in affected individuals is excreted by the kidney at a rate practically equivalent to the glomerular filtration rate (GFR). Whether ASA itself causes a degree of toxicity due to hepatocellular accumulation is unknown; such an effect could help explain hyperammonemia development in affected individuals. Regardless, the name of the disease is derived from the rapid clearance of ASA in urine, although elevated levels of ASA can be found in plasma. ASA lyase deficiency is associated with high mortality and morbidity rates. Symptoms of ASA lyase deficiency include anorexia, irritability rapid breathing, lethargy and vomiting. Extreme symptoms include coma and cerebral edema. [HMDB] |
|---|
| CAS Number | 2387-71-5 |
|---|
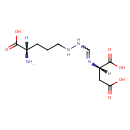
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 2-(N(omega)-L-arginine)succinate | hmdb | | 2-(N(omega)-L-arginine)succinic acid | hmdb | | 2-(N(omega)-L-arginino)succinate | hmdb | | 2-(N(omega)-L-arginino)succinic acid | hmdb | | 2-(Nomega-L-arginino)succinate | Kegg | | 2-(Nomega-L-arginino)succinic acid | Generator | | 2-(Nw-L-arginino)butanedioate | hmdb | | 2-(Nw-L-arginino)butanedioic acid | hmdb | | Argininosuccinate | hmdb | | Argininosuccinic acid | hmdb | | Arginosuccinate | hmdb | | Arginosuccinic acid | hmdb | | ASA | hmdb | | L-Argininosuccinate | hmdb | | L-Argininosuccinic acid | hmdb | | L-Arginosuccinate | hmdb | | L-Arginosuccinic acid | hmdb | | N-(((4-amino-4-carboxybutyl)amino)iminomethyl)-L-Aspartate | hmdb | | N-(((4-amino-4-carboxybutyl)amino)iminomethyl)-L-Aspartic acid | hmdb | | N-(L-arginino) succinate | hmdb | | N-(L-arginino) succinic acid | hmdb | | N-(L-Arginino)succinate | hmdb | | N-(L-Arginino)succinic acid | hmdb | | N-[(4-amino-4-carboxybutyl)amidino]-L-Aspartate | hmdb | | N-[(4-amino-4-carboxybutyl)amidino]-L-Aspartic acid | hmdb | | N-[[(4-amino-4-carboxybutyl)amino]iminomethyl]-L-Aspartate | hmdb | | N-[[(4-amino-4-carboxybutyl)amino]iminomethyl]-L-Aspartic acid | hmdb | | N(omega)-(L-arginino)succinate | hmdb | | N(omega)-(L-arginino)succinic acid | hmdb |
|
|---|
| Chemical Formula | C10H18N4O6 |
|---|
| IUPAC name | (2S)-2-[({2-[(4S)-4-amino-4-carboxybutyl]hydrazin-1-yl}methylidene)amino]butanedioic acid |
|---|
| InChI Identifier | InChI=1S/C10H18N4O6/c11-6(9(17)18)2-1-3-13-14-5-12-7(10(19)20)4-8(15)16/h5-7,13H,1-4,11H2,(H,12,14)(H,15,16)(H,17,18)(H,19,20)/t6-,7-/m0/s1 |
|---|
| InChI Key | WSQWJAOQSDPYTD-BQBZGAKWSA-N |
|---|
| Isomeric SMILES | [H][C@](N)(CCCNN=CN[C@@]([H])(CC(O)=O)C(O)=O)C(O)=O |
|---|
| Average Molecular Weight | 290.2731 |
|---|
| Monoisotopic Molecular Weight | 290.122634328 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as aspartic acid and derivatives. Aspartic acid and derivatives are compounds containing an aspartic acid or a derivative thereof resulting from reaction of aspartic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Aspartic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aspartic acid or derivatives
- L-alpha-amino acid
- Alpha-amino acid
- Tricarboxylic acid or derivatives
- Amino acid
- Carboxylic acid amidrazone
- Formamidine
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboxylic acid
- Amidine
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 5 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |