| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:36 UTC |
|---|
| Update date | 2017-01-19 02:36:18 UTC |
|---|
| FoodComEx ID | PC000272 |
|---|
| FoodDB Record | FDB005421 |
|---|
| Chemical Information |
|---|
| Name | Methylguanidine |
|---|
| Description | Methylguanidine (MG) is a guanidine compound deriving from protein catabolism. It is also a product of putrefaction. Methylguanidine is a suspected uraemic toxin that accumulates in renal failure, however it also exhibits anti-inflammatory effects. Methylguanidine is synthesized from creatinine concomitant with the synthesis of hydrogen peroxide from endogenous substrates in peroxisomes. Recent evidence suggests that methylguanidine significantly inhibits iNOS activity and TNF- release. This means that methylguandine can attenuate the degree of inflammation and tissue damage associated with endotoxic shock. Methylguanidine is found in loquat and apple. |
|---|
| CAS Number | 471-29-4 |
|---|
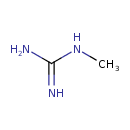
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1-methylguanidine | biospider | | Guanidine, methyl- | biospider | | Methylguanidin | biospider | | Methylguanidine | biospider | | MGX | biospider | | Monomethyl guanidin | biospider | | Monomethylguanidine | biospider | | N-methylguanidine | biospider | | N1-Methylguanidine | biospider |
|
|---|
| Chemical Formula | C2H7N3 |
|---|
| IUPAC name | N-methylguanidine |
|---|
| InChI Identifier | InChI=1S/C2H7N3/c1-5-2(3)4/h1H3,(H4,3,4,5) |
|---|
| InChI Key | CHJJGSNFBQVOTG-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CNC(N)=N |
|---|
| Average Molecular Weight | 73.0971 |
|---|
| Monoisotopic Molecular Weight | 73.063997239 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as guanidines. Guanidines are compounds containing a guanidine moiety, with the general structure (R1R2N)(R3R4N)C=N-R5. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Guanidines |
|---|
| Direct Parent | Guanidines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Guanidine
- Carboximidamide
- Organopnictogen compound
- Hydrocarbon derivative
- Imine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | 1.78 mg/mL at 20 oC | GREENWALD,I (1926) |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |