| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:35 UTC |
|---|
| Update date | 2017-01-19 02:36:18 UTC |
|---|
| FoodComEx ID | PC000266 |
|---|
| FoodDB Record | FDB001976 |
|---|
| Chemical Information |
|---|
| Name | L-Cystathionine |
|---|
| Description | Isolated from Phallus impudicus (common stinkhorn)
Cystathionine is a dipeptide formed by serine and homocysteine. Cystathioninuria is a prominent manifestation of vitamin-B6 deficiency. The transsulfuration of methionine yields homocysteine, which combines with serine to form cystathionine, the proximate precursor of cysteine through the enzymatic activity of cystathionase. In conditions in which cystathionine gamma-synthase or cystathionase is deficient, for example, there is cystathioninuria. Although cystathionine has not been detected in normal human serum or plasma by most conventional methods, gas chromatographic/mass spectrometric methodology detected a mean concentration of cystathionine in normal human serum of 140 nM, with a range of 65 to 301 nM.567 Cystathionine concentrations in CSF have been 10, 1, and 0.5 uM, and "not detected." Only traces (i.e., <1 uM) of cystathionine are present in normal CSF.587. gamma-Cystathionase deficiency provided the first instance in which, in a human, the major biochemical abnormality due to a defined enzyme defect was clearly shown to be alleviated by administration of large doses of pyridoxine. The response in gamma-cystathionase-deficient patients is not attributable to correction of a preexisting deficiency of this vitamin. (OMMBID, Chap. 88); Cystathionine is an intermediate in the synthesis of cysteine. L-Cystathionine is found in many foods, some of which are spirulina, greenthread tea, gooseberry, and quinoa. |
|---|
| CAS Number | 56-88-2 |
|---|
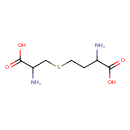
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (R)-S-(2-amino-2-carboxyethyl)-L-Homocysteine | biospider | | [R-(R*,s*)]-2-amino-4-[(2-amino-2-carboxyethyl)thio]-butanoate | HMDB | | [R-(R*,s*)]-2-amino-4-[(2-amino-2-carboxyethyl)thio]-butanoic acid | HMDB | | Cystathionine | biospider | | Cystathionine (6CI,7CI) | biospider | | Cystathionine, l- | biospider | | Cystathionine, L- (8CI) | biospider | | L-(+)-cystathionine | biospider | | L-Cystathionine | db_source | | L-Homocysteine, S-((2R)-2-amino-2-carboxyethyl)- | biospider | | L-Homocysteine, S-(2-amino-2-carboxyethyl)-, (R)- | biospider | | L-Homocysteine, S-[(2R)-2-amino-2-carboxyethyl]- | biospider | | L-Homocysteine, S-[(2R)-2-amino-2-carboxyethyl]- (9CI) | biospider | | S-(b-amino-b-Carboxyethyl)homocysteine | Generator | | S-(beta-amino-beta-carboxyethyl)homocysteine | biospider | | S-(β-amino-β-carboxyethyl)homocysteine | Generator | | S-[(2R)-2-amino-2-carboxyethyl]-L-Homocysteine | biospider |
|
|---|
| Chemical Formula | C7H14N2O4S |
|---|
| IUPAC name | 2-amino-4-[(2-amino-2-carboxyethyl)sulfanyl]butanoic acid |
|---|
| InChI Identifier | InChI=1S/C7H14N2O4S/c8-4(6(10)11)1-2-14-3-5(9)7(12)13/h4-5H,1-3,8-9H2,(H,10,11)(H,12,13) |
|---|
| InChI Key | ILRYLPWNYFXEMH-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NC(CCSCC(N)C(O)=O)C(O)=O |
|---|
| Average Molecular Weight | 222.262 |
|---|
| Monoisotopic Molecular Weight | 222.067427636 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as cysteine and derivatives. Cysteine and derivatives are compounds containing cysteine or a derivative thereof resulting from reaction of cysteine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Cysteine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cysteine or derivatives
- Alpha-amino acid
- Thia fatty acid
- Dicarboxylic acid or derivatives
- Fatty acid
- Fatty acyl
- Amino acid
- Carboxylic acid
- Thioether
- Sulfenyl compound
- Dialkylthioether
- Organic nitrogen compound
- Organopnictogen compound
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Organic oxygen compound
- Amine
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 282-283° dec. | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 200 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Cayman Chemical | 16061 |
|---|
| Toronto Research Chemicals | C989500 |
|---|