| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:27 UTC |
|---|
| Update date | 2017-01-19 02:36:18 UTC |
|---|
| FoodComEx ID | PC000252 |
|---|
| FoodDB Record | FDB001037 |
|---|
| Chemical Information |
|---|
| Name | 4-Aminobenzoic acid |
|---|
| Description | p-Aminobenzoic acid, also known as PABA or p-aminobenzoate, belongs to the class of organic compounds known as aminobenzoic acids. These are benzoic acids containing an amine group attached to the benzene moiety. p-Aminobenzoic acid is a moderately basic compound (based on its pKa). p-Aminobenzoic acid exists in all living species, ranging from bacteria to humans. Outside of the human body, p-Aminobenzoic acid is found, on average, in the highest concentration within pineapples. p-Aminobenzoic acid has also been detected, but not quantified in, milk (cow) and rices. This could make p-aminobenzoic acid a potential biomarker for the consumption of these foods. An aminobenzoic acid in which the amino group is para to the carboxy group. |
|---|
| CAS Number | 150-13-0 |
|---|
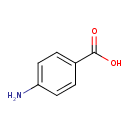
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| «gamma»-aminobenzoic acid | biospider | | 1-Amino-4-carboxybenzene | biospider | | 4-amino-Benzoate | Generator | | 4-AMINO-BENZOIC ACID | biospider | | 4-Aminobenzoate | biospider | | 4-Aminobenzoesaeure | ChEBI | | 4-Carboxyaniline | biospider | | 4-Carboxyphenylamine | biospider | | ABEE | biospider | | Acido p-aminobenzoico | biospider | | Acidum paraminobenzoicum | biospider | | Actipol | biospider | | Amben | biospider | | Aminobenzoate | biospider | | Aminobenzoate acid | biospider | | Aminobenzoic acid | biospider | | Aminobenzoic acid (usp) | biospider | | Aminobenzoic acid, para | biospider | | Aminobenzoic acid, USAN | db_source | | Aniline-4-carboxylate | biospider | | Aniline-4-carboxylic acid | biospider | | Anti-chromotrichia factor | biospider | | Anticanitic vitamin | biospider | | Anticantic vitamin | biospider | | Antichromotrichia factor | biospider | | Bacterial vitamin H1 | biospider | | Benzoic acid, 4-amino | biospider | | Benzoic acid, 4-amino- | biospider | | Benzoic acid, p-amino- | biospider | | Chromotrichia factor | biospider | | g-Aminobenzoate | Generator | | g-Aminobenzoic acid | Generator | | Gamma-aminobenzoate | biospider | | Gamma-aminobenzoic acid | biospider | | Hachemina | biospider | | Kyselina P-aminobenzoova | HMDB | | M-aminobenzonitrile | biospider | | P-amino-benzoate | biospider | | P-amino-benzoic acid | biospider | | P-aminobenzoate | biospider | | P-Aminobenzoesaeure | ChEBI | | P-aminobenzoic acid | biospider | | P-carboxyaniline | biospider | | P-carboxyphenylamine | biospider | | PAB | biospider | | PABA | db_source | | Pabacyd | biospider | | Pabafilm | biospider | | Pabagel | biospider | | Pabamine | biospider | | Pabanol | biospider | | Papacidum | biospider | | Para-aminobenzoate | biospider | | Para-aminobenzoic acid | biospider | | Paraminol | biospider | | Paranate | biospider | | Potaba | biospider | | Romavit | biospider | | Rvpaba | biospider | | Rvpaba lipstick | biospider | | Rvpaba lipstick (TN) | biospider | | Sodium Aminobenzoate (4-Aminobenzoic Acid) | biospider | | Sunbrella | biospider | | Super shade BY coppertone | biospider | | Trichochromogenic factor | biospider | | Trochromogenic factor | biospider | | Vitamin BX | biospider | | Vitamin H' | db_source | | γ-aminobenzoate | Generator | | γ-aminobenzoic acid | Generator |
|
|---|
| Chemical Formula | C7H7NO2 |
|---|
| IUPAC name | 4-aminobenzoic acid |
|---|
| InChI Identifier | InChI=1S/C7H7NO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4H,8H2,(H,9,10) |
|---|
| InChI Key | ALYNCZNDIQEVRV-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NC1=CC=C(C=C1)C(O)=O |
|---|
| Average Molecular Weight | 137.136 |
|---|
| Monoisotopic Molecular Weight | 137.047678473 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as aminobenzoic acids. These are benzoic acids containing an amine group attached to the benzene moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Aminobenzoic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aminobenzoic acid
- Benzoic acid
- Benzoyl
- Aniline or substituted anilines
- Amino acid or derivatives
- Amino acid
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Amine
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | 0.83 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 6.11 mg/mL at 30 oC | YALKOWSKY,SH & HE,Y (2003) |
|---|
| Melting Point | Mp 188-188.5° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 500 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | F369 |
|---|
| AKSci | J90095 |
|---|
| Glentham | GM6679 |
|---|
| Glentham | GM2145 |
|---|
| MetaSci | HMDB0001392 |
|---|
| Sigma-Aldrich | HMDB0001392 |
|---|
| Toronto Research Chemicals | A591500 |
|---|