| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:24 UTC |
|---|
| Update date | 2017-01-19 02:36:17 UTC |
|---|
| FoodComEx ID | PC000241 |
|---|
| FoodDB Record | FDB022408 |
|---|
| Chemical Information |
|---|
| Name | 2-Phenylaminoadenosine |
|---|
| Description | Selective A2 adenosine receptor agonist; potent coronary vasodilator; weak inhibitor of adenosine uptake by rat cerebral cortical synaptosomes; used as a vasodilator agent; is a potent anti-inflammatory agent, acting at its four G protein coupled receptors. Topical treatment of adenosine to foot wounds in diabetes mellitus has been shown in lab animals to drastically increase tissue repair and reconstruction. Topical administration of adenosine for use in wound healing deficiencies and diabetes mellitus in humans is currently under clinical investigation.
Adenosine is a nucleoside comprised of adenine attached to a ribose (ribofuranose) moiety via a beta-N9-glycosidic bond. [HMDB] |
|---|
| CAS Number | 53296-10-9 |
|---|
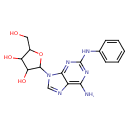
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 6-amino-2-phenylamino-9-b-D-ribofuranosyl-9H-purine | hmdb | | 6-amino-2-phenylamino-9-beta-delta-ribofuranosyl-9H-purine | hmdb |
|
|---|
| Chemical Formula | C16H18N6O4 |
|---|
| IUPAC name | 2-[6-amino-2-(phenylamino)-9H-purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol |
|---|
| InChI Identifier | InChI=1S/C16H18N6O4/c17-13-10-14(21-16(20-13)19-8-4-2-1-3-5-8)22(7-18-10)15-12(25)11(24)9(6-23)26-15/h1-5,7,9,11-12,15,23-25H,6H2,(H3,17,19,20,21) |
|---|
| InChI Key | SCNILGOVBBRMBK-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NC1=C2N=CN(C3OC(CO)C(O)C3O)C2=NC(NC2=CC=CC=C2)=N1 |
|---|
| Average Molecular Weight | 358.3519 |
|---|
| Monoisotopic Molecular Weight | 358.138953094 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as purine nucleosides. Purine nucleosides are compounds comprising a purine base attached to a ribosyl or deoxyribosyl moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Purine nucleosides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Purine nucleosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Purine nucleoside
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- N-substituted imidazole
- Monosaccharide
- Monocyclic benzene moiety
- Pyrimidine
- Benzenoid
- Imidazole
- Azole
- Tetrahydrofuran
- Heteroaromatic compound
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Primary amine
- Amine
- Alcohol
- Organic nitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 50 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | liquid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 7561AB |
|---|
| Toronto Research Chemicals | P319530 |
|---|