| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:19 UTC |
|---|
| Update date | 2017-01-19 02:36:17 UTC |
|---|
| FoodComEx ID | PC000226 |
|---|
| FoodDB Record | FDB010534 |
|---|
| Chemical Information |
|---|
| Name | 4-Hydroxyphenylacetic acid |
|---|
| Description | Constituent of sweet clover (Melilotus officinalis) and yeast
An oxidative deaminated metabolite of p-tyramineand is also a metabolite of tyrosine via enteric bacteria. The bacterial origin of this compound was confirmed by the finding that this compound in urine decreased significantly after the use of the antibiotic neomycin. 4-Hydroxyphenylacetic acid is found in many foods, some of which are dandelion, evening primrose, grape wine, and beer. |
|---|
| CAS Number | 156-38-7 |
|---|
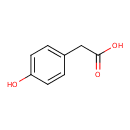
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (4-hydroxy-phenyl)-acetate | biospider | | (4-hydroxy-phenyl)-acetic acid | biospider | | (4-Hydroxy-phenyl)-essigsaeure | HMDB | | (4-hydroxyphenyl)acetate | biospider | | (4-hydroxyphenyl)acetic acid | biospider | | (p-hydroxyphenyl)-acetate | biospider | | (p-hydroxyphenyl)-acetic acid | biospider | | (p-hydroxyphenyl)acetate | biospider | | (p-hydroxyphenyl)acetic acid | biospider | | 3pcg | biospider | | 4-Carboxymethylphenol | biospider | | 4-HPA | biospider | | 4-Hydroxy-Benzeneacetate | biospider | | 4-Hydroxy-Benzeneacetic acid | biospider | | 4-Hydroxybenzeneacetate | biospider | | 4-Hydroxybenzeneacetic acid | biospider | | 4-Hydroxybenzeneacetic acid, 9CI | db_source | | 4-hydroxyphenyl acetate | biospider | | 4-Hydroxyphenyl-acetic acid | HMDB | | 4-hydroxyphenylacetate | biospider | | Acetic acid, (p-hydroxyphenyl)- | biospider | | Benzeneacetic acid, 4-hydroxy- | biospider | | DL-para-hydroxyphenylacetic acid | biospider | | p-Hydroxy-a-toluic acid | db_source | | P-hydroxyphenyl acetic acid | biospider | | P-hydroxyphenylacetate | biospider | | P-hydroxyphenylacetic acid | biospider | | Parahydroxy phenylacetate | biospider | | Parahydroxy phenylacetic acid | biospider | | Parahydroxyphenylacetate | biospider |
|
|---|
| Chemical Formula | C8H8O3 |
|---|
| IUPAC name | 2-(4-hydroxyphenyl)acetic acid |
|---|
| InChI Identifier | InChI=1S/C8H8O3/c9-7-3-1-6(2-4-7)5-8(10)11/h1-4,9H,5H2,(H,10,11) |
|---|
| InChI Key | XQXPVVBIMDBYFF-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OC(=O)CC1=CC=C(O)C=C1 |
|---|
| Average Molecular Weight | 152.1473 |
|---|
| Monoisotopic Molecular Weight | 152.047344122 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as 1-hydroxy-2-unsubstituted benzenoids. These are phenols that are unsubstituted at the 2-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | 1-hydroxy-2-unsubstituted benzenoids |
|---|
| Direct Parent | 1-hydroxy-2-unsubstituted benzenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | 0.75 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 60.7 mg/mL at 25 oC | GRACIN,S & RASMUSON,AC (2002) |
|---|
| Melting Point | Mp 148-150° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | A096 |
|---|
| AKSci | J94171 |
|---|
| MetaSci | HMDB0000020 |
|---|
| Sigma-Aldrich | HMDB0000020 |
|---|
| Toronto Research Chemicals | H949060 |
|---|